Abstract
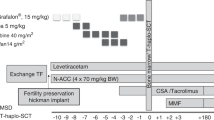
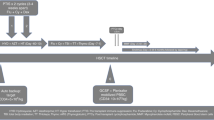
Peripheral blood stem cells (PBSC) were mobilized in 130 patients with autoimmune diseases undergoing autologous hematopoietic stem cell transplantation using cyclophosphamide 2 g/m2 and either granulocyte colony-stimulating factor (G-CSF) 5 mcg/kg/day (for systemic lupus erythematosus (SLE) and secondary progressive multiple sclerosis, SPMS) or G-CSF 10 mcg/kg/day (for relapsing remitting multiple sclerosis (RRMS), Crohn's disease (CD), systemic sclerosis (SSc), and other immune-mediated disorders). Mobilization-related mortality was 0.8% (one of 130) secondary to infection. Circulating peripheral blood (PB) CD34+ cells/μl differed significantly by disease. Collected CD34+ cells/kg/apheresis and overall collection efficiency was significantly better using Spectra apheresis device compared to the Fenwall CS3000 instrument. Patients with SLE and RRMS achieved the lowest and the highest CD34+ cell yields, respectively. Ex vivo CD34+ cell selection employing Isolex 300iv2.5 apparatus was significantly more efficient compared to CEPRATE CS device. Circulating PB CD34+ cells/μl correlated positively with initial CD34+ cells/kg/apheresis and enriched product CD34+ cells/kg. Mean WBC and platelet engraftment (ANC>0.5 × 109/l and platelet count >20 × 109/l) occurred on days 9 and 11, respectively. Infused CD34+ cell/kg dose showed significant direct correlation with faster white blood cell (WBC) and platelet engraftment. When adjusted for CD34+ cell/kg dose, patients treated with a myeloablative regimen had significantly slower WBC and platelet recovery compared to non-myeloablative regimens.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Fruehauf S, Haas R, Conradt C, Murea S, Witt B, Mohle R et al. Peripheral blood progenitor cell (PBPC) counts during steady-state hematopoiesis allow to estimate the yield of mobilized PBPC after filgrastim (R-metHuG-CSF)-supported cytotoxic chemotherapy. Blood 1995; 85: 2619–2626.
Roberts AW, Begley CG, Grigg AP, Basser RL . Do steady-state peripheral blood progenitor cell (PBPC) counts predict the yield of PBPC mobilized by filgrastim alone? Blood 1995; 86: 2451.
Husson B, Ravoet C, Dehon M, Wallef G, Hougardy N, Delannoy A . Predictive value of the steady-state peripheral blood progenitor cell (PBPC) counts for the yield of PBPC collected by leukapheresis after mobilization by granulocyte colony-stimulating factor (G-CSF) alone or chemotherapy and G-CSF. Blood 1996; 87: 3526–3528.
Breems DA, van Hennik PB, Kusadasi N, Boudewijn A, Cornelissen JJ, Sonneveld P et al. Individual stem cell quality in leukapheresis products is related to the number of mobilized stem cells. Blood 1996; 87: 5370–5378.
Koc ON, Gerson SL, Cooper BW, Laughlin M, Meyerson H, Kutteh L et al. Randomized cross-over trial of progenitor-cell mobilization: high-dose cyclophosphamide plus granulocyte colony-stimulating factor (G-CSF) versus granulocyte-macrophage colony-stimulating factor plus G-CSF. J Clin Oncol 2000; 18: 1824–1830.
Akard L . Optimum methods to mobilize stem cells. J Clin Oncol 2000; 18: 3063.
Chabannon C, Le Corroller A-G, Viret F, Eillen C, Faucher C, Moatti J-P et al. Cost-effectiveness of repeated aphereses in poor mobilizers undergoing high-dose chemotherapy and autologous hematopoietic cell transplantation. Leukemia 2003; 17: 811–820.
Haas R, Mohle R, Fruhauf S, Goldschmidt H, Witt B, Flentje M et al. Patient characteristics associated with successful mobilizing and autografting of peripheral blood progenitor cells in malignant lymphoma. Blood 1994; 83: 3787–3794.
Narayanasami U, Kanteti R, Morelli J, Klekar A, Al-Olama A, Keating C et al. Randomized trial of filgrastim versus chemotherapy and filgrastim mobilization of hematopoietic progenitor cells for rescue in autologous transplantation. Blood 2001; 98: 2059–2064.
Deliliers GL, Annaloro C, Marconi M, Soligo D, Morandi P, Luchesini C et al. Harvesting of autologous blood stem cells after a mobilising regimen with low-dose cyclophosphamide. Leuk Lymph 2002; 43: 1957–1960.
Mollee P, Pereira D, Nagy T, Song K, Saragosa R, Keating A et al. Cyclophosphamide, etoposide and G- CSF to mobilize peripheral blood stem cells for autologous stem cell transplantation in patients with lymphoma. Bone Marrow Tranpslant 2002; 30: 272–278.
Petzer AL, Hochenburger E, Haun M, Duba HC, Grunewald K, Hoflehner E et al. High-dose hydroxyurea plus G-CSF mobilize BCR-ABL-negative progenitor cells (CFC, LTC-IC) into the blood of newly diagnosed CML patients at any time of hematopoietic regeneration. J Hematother Stem Cell Res 2002; 11: 293–300.
Weaver CH, Birch R, Greco FA, Schwartzberg L, McAneny B, Moore M et al. Mobilization and harvesting of peripheral blood stem cells: randomized evaluations of different doses of filgrastim. Br J Haematol 1998; 100: 338–347.
Yanovich S, Mitsky P, Cornetta K, Maziarz RT, Rosenfeld C, Krause DS et al. Transplantation of CD34+ peripheral blood cells selected using a fully automated immunomagnetic system in patients with high-risk breast cancer: results of a prospective randomized multicenter clinical trial. Bone Marrow Transplant 2000; 25: 1165–1174.
Meehan KR, Slack R, Gehan E, Herscowitz HB, Areman EM, Ebadi M et al. Mobilization of peripheral blood stem cells with paclitaxel and rhG-CSF in high-risk breast cancer patients. J Hematother Stem Cell Res 2002; 11: 415–421.
Prosper F, Sola C, Hornedo J, Arbona C, Menendez P, Orfao A et al. Mobilization of peripheral blood progenitor cells with a combination of cyclophosphamide, r-metHuCSF and filgrastim in patients with breast cancer previously treated with chemotherapy. Leukemia 2003; 17: 437–441.
Martin-Murea S, Voso MT, Hohaus S, Pforsich M, Fruehauf S, Golschmidt H et al. The dose of granulocyte colony-stimulating factor administered following cytotoxic chemotherapy is not related to the rebound level of circulating CD34+ haematopoietic progenitor cells during marrow recovery. Br J Haematol 1998; 101: 582–585.
Morris CL, Siegel E, Barlogie B, Cottler-Fox M, Lin P, Fassas A et al. Mobilization of CD34+ cells in elderly patients (>70 years) with multiple myeloma: influence of age, prior therapy, platelet count and mobilization regimen. Br J Haematol 2003; 120: 413–423.
Sevilla J, Gonzalez-Vicent M, Madero L, Garcia-Sanchez F, Diaz MA . Granulocyte colony-stimulating factor alone at 12 μg/kg twice a day for 4 days for peripheral blood progenitor cell priming in pediatric patients. Bone Marrow Transplant 2002; 30: 417–420.
Ketterer N, Salles G, Raba M, Espinouse D, Sonet A, Tremisi P et al. High CD34+ cell counts decrease hematologic toxicity of autologous peripheral blood progenitor cell transplantation. Blood 1998; 91: 3148–3155.
Gordon PR, Leimig T, Mueller I, Babarin-Dorner A, Holladay MA, Houston J et al. A large-scale method for T cell depletion: towards graft engineering of mobilized peripheral blood stem cells. Bone Marrow Transplant 2002; 30: 69–74.
Dunbar CE, Cottler-Fox M, O'Shaughnessy JA, Doren S, Carter C, Berenson R et al. Retrovirally marked CD34-enriched peripheral blood and bone marrow cells contribute to long-term engraftment after autologous transplantation. Blood 1995; 85: 3048–3057.
De Boer F, Drager AM, Van Haperen MJAM, Van der Wall E, Kessler F, Huijgens PC et al. The phenotypic profile of CD34-positive peripheral blood stem cells in different mobilization regimens. Br J Haematol 2000; 111: 1138–1144.
Sutherland DR, Anderson L, Keeney M, Nayar R, Chin-Yee I . The ISHAGE guidelines for CD34+ cell determination by flow cytometry. J Hematother 1996; 5: 213–226.
Shpall EJ, Champlin R, Glaspy JA . Effect of CD34+ peripheral blood progenitor cell dose on hematopoietic recovery. Biol Blood Marrow Transplant 1998; 4: 84–92.
Gryn J, Shadduck RK, Lister J, Zeigler ZR, Raymond JM . Factors affecting purification of CD34+ peripheral blood stem cells using Baxter Isolex 300i. J Hematother Stem Cell Res 2002; 11: 719–730.
Fruehauf S, Seggewiss R . It's moving day: factors affecting peripheral blood stem cell mobilization and strategies for improvement (review). Br J Haematol 2003; 122: 360–375.
Burt RK, Verda L, Oyama Y, Statkute L, Slavin S . Non-myeloablative stem cell transplantation for autoimmune diseases. Springer Semin Immunopathol 2004; 26: 57–69.
Sykes M, Nikolic B . Treatment of severe autoimmune disease by stem-cell transplantation. Nature 2005; 435: 620–627.
Popat U, Krance R . Haematopoietic stem cell transplantation for autoimmune disorders: the American perspective. Br J Haematol 2004; 126: 637–649.
Hough RE, Snowden JA, Wulffraat NM . Haemopoietic stem cell transplantation in autoimmune diseases: a European perspective. Br J Haematol 2005; 128: 432–459.
Burt RK, Marmont A, Oyama Y, Slavin S, Arnold R, Hiepe F et al. Randomized controlled trials of autologous hematopoietic stem cell transplantation for autoimmune diseases: the evolution from myeloablative to lymphoablative transplant regimens. Arthr Rheum 2006; 54: 3750–3760.
McGonagle D, Rawstron A, Richards S, Isaacs J, Bird H, Jack A et al. A phase 1 study to address the safety and efficacy of granulocyte colony-stimulating factor for the mobilization of hematopoietic progenitor cells in active rheumatoid arthritis. Arthr Rheum 1997; 40: 1838–1842.
Snowden JA, Biggs JC, Miliken ST, Fuller A, Staniforth D, Passuello F et al. A randomized, blinded, placebo-controlled, dose escalation study of the tolerability and efficacy of filgrastim for hematopoietic stem cell mobilization in patients with severe active rheumatoid arthritis. Bone Marrow Transplant 1998; 22: 1035–1041.
Breban M, Dougados M, Picard F, Zompi S, Marolleau J-P, Bocaccio C et al. Intensified-dose (4 g/m2) cyclophosphamide and granulocyte colony-stimulating factor administration for hematopoietic stem cell mobilization in refractory rheumatoid arthritis. Arthr Rheum 1999; 42: 2275–2280.
Locatelli F, Perotti C, Torretta L, Maccario R, Montagna D, Ravelli A et al. Mobilization and selection of peripheral blood hematopoietic progenitors in children with systemic sclerosis. Haematologica 1999; 84: 839–843.
Burt RK, Fassas A, Snowden JA, van Laar JM, Kozak T, Wulffraat NM et al. Collection of hematopoietic stem cells from patients with autoimmune diseases. Bone Marrow Transplant 2001; 28: 1–12.
Openshaw H, Stuve O, Antel JP, Nash R, Lund BT, Weiner LP et al. Multiple sclerosis flares associated with recombinant granulocyte colony-stimulating factor. Neurology 2000; 54: 2147–2150.
Euler HH, Harten P, Zeuner RA, Schwab UM . Recombinant human granulocyte colony stimulating factor in patients with systemic lupus erythematosus associated neutropenia and refractory infections. J Rheumatol 1997; 24: 2153–2157.
Gottenberg JE, Roux S, Desmoulins F, Clerc D, Mariette X . Granulocyte colony-stimulating factor therapy resulting in a flare of systemic lupus erythematosus: comment on the article by Yang and Hamilton. Arthr Rheum 2001; 44: 2458–2460.
Hohaus S, Martin H, Wassmann B, Egerer G, Haus U, Farber L et al. Recombinant human granulocyte and granulocyte-macrophage colony-stimulating factor (G-CSF and GM-CSF) administered following cytotoxic chemotherapy have a similar ability to mobilize peripheral blood stem cells. Bone Marrow Transplant 1998; 22: 625–630.
Ketterer N, Salles G, Moullet I, Dumontet C, ElJaafari-Corbin A, Tremisi P et al. Factors associated with successful mobilization of peripheral blood progenitor cells in 200 patients with lymphoid malignancies. Br J Haematol 1998; 103: 235–242.
D'Arena G, Musto P, Di Mauro L, Cascavilla N, Iacono ND, Scalzulli PR et al. Predictive parameters for mobilized peripheral blood CD34+ progenitor cell collection in patients with hematological malignancies. Am J Hematol 1998; 58: 255–262.
Demirer T, Buckner CD, Storer B, Lilleby K, Rowley S, Clift R et al. Effect of different chemotherapy regimens on peripheral-blood stem-cell collections in patients with breast cancer receiving granulocyte colony-stimulating factor. J Clin Oncol 1997; 15: 684–690.
Tarella C, Zallio F, Caracciolo D, Cherasco C, Bondesan P, Gavarotti P et al. Hematopoietic progenitor cell mobilization and harvest following an intensive chemotherapy debulking in indolent lymphoma patients. Stem Cells 1999; 17: 55–61.
Sautois B, Fraipont V, Baudoux E, Fassotte MF, Hermanne JP, Jerusalem G et al. Peripheral blood progenitor cell collections in cancer patients: analysis of factors affecting the yields. Haematologica 1999; 84: 342–349.
Kudo Y, Minegishi M, Saito N, Itoh T, Fushimi J, Takahashi H et al. The absolute number of peripheral blood CD34+ cells predicts a timing for apheresis and progenitor cell yield in patients with hematologic malignancies and solid tumors. Tohoku J Exp Med 2003; 199: 111–118.
Mehta J, Singhal S, Gordon L, Tallman M, Williams S, Luyun R et al. Cobe Spectra is superior to Fenwal CS 3000 Plus for collection of hematopoietic stem cells. Bone Marrow Transplantation 2002; 29: 563–567.
Hitzler WE, Wolf S, Runkel S, Kunz-Kostomanolakis M . Comparison of intermittent- and continuous-flow cell separators for the collection of autologous peripheral blood progenitor cells in patients with hematologic malignancies. Transfusion 2001; 41: 1562–1566.
Despres D, Flohr T, Uppenkamp M, Baldus M, Hoffmann M, Huber C et al. CD34+ cell enrichment for autologous peripheral blood stem cell transplantation by use of the CliniMACS device. J Hematother Stem Cell Res 2000; 9: 557–564.
Schumm M, Lang P, Taylor G, Kuci S, Klingebiel T, Buhring HJ et al. Isolation of highly purified autologous and allogeneic peripheral CD34+ cells using the CliniMACS device. J Hematother 1999; 8: 209–218.
Weaver C, Hazelton B, Birch R, Palmer P, Allen C, Schwartzberg L et al. An analysis of engraftment kinetics as a function of the CD34 content of peripheral blood progenitor cell collections in 692 patients after the administration of myeloablative chemotherapy. Blood 1995; 86: 3961–3969.
Kim I, Yoon SS, Lee K-H, Keam B, Kim TM, Kim J-S et al. Comparative outcomes of reduced intensity and myeloablative allogeneic hematopoietic stem cell transplantation in patients under 50 with hematologic malignancies. Clin Transplant 2006; 20: 496–503.
Vela-Ojeda J, Garcia-Ruiz Esparza MA, Tripp-Villanueva F, Ayala-Sanchez M, Delgado-Lamas JL, Garces-Ruiz O et al. Allogeneic peripheral blood stem cell transplantation using reduced intensity versus myeloablative conditioning regimens for the treatment of leukemia. Stem Cells Dev 2004; 13: 571–578.
Acknowledgements
We thank the Lupus Foundation of America, Illinois Chapter for their financial and patient support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Statkute, L., Verda, L., Oyama, Y. et al. Mobilization, harvesting and selection of peripheral blood stem cells in patients with autoimmune diseases undergoing autologous hematopoietic stem cell transplantation. Bone Marrow Transplant 39, 317–329 (2007). https://doi.org/10.1038/sj.bmt.1705579
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.bmt.1705579
Keywords
This article is cited by
-
Hypersplenism in liver disease and SLE revisited: current evidence supports an active rather than passive process
BMC Hematology (2016)
-
Autologous hematopoietic stem cell transplantation in multiple sclerosis: 20 years of experience
Neurological Sciences (2016)
-
Management of Neuropsychiatric Systemic Lupus Erythematosus: Current Approaches and Future Perspectives
Drugs (2016)
-
Five-year disease-free survival among stage II-IV breast cancer patients receiving FAC and AC chemotherapy in phase II clinical trials of Panagen
BMC Cancer (2016)
-
Haematopoietic SCT in severe autoimmune diseases: updated guidelines of the European Group for Blood and Marrow Transplantation
Bone Marrow Transplantation (2012)