Abstract
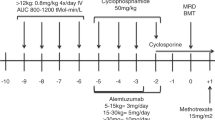
Allogenic hematopoietic stem cell transplant is the only curative option for symptomatic sickle cell disease (SCD). HLA haploidentical related donor transplants are associated with high graft failure rates. We conceptualized a novel protocol (APOLLO protocol) using pre-transplant immune and myelosuppression (PTIS) using fludarabine, cyclophosphamide, and dexamethasone followed by augmented John Hopkins protocol by adding thiotepa to conditioning. Twenty-five consecutive patients suffering from symptomatic SCD were enrolled into the study. We added upfront plerixafor to granulocyte colony stimulating factor (GCSF) for mobilization of healthy donors. Graft versus host disease (GvHD) prophylaxis was done using post-transplant cyclophosphamide, sirolimus, and mycophenolate mofetil. Graft failure was not seen in any of our patients. Five patients developed acute grade II/IV GvHD (4 classical acute, 1 late onset), 3 had limited chronic GvHD. Out of 25 evaluable patients, 22 are alive and disease free, making an overall survival (OS) and disease-free survival (DFS) of 88% with a median follow up of 485 days (range 198–802). T-cell-replete haploidentical transplant with PTIS, augmented John Hopkins conditioning and plerixafor based mobilization is a safe and effective way of treating patients suffering from SCD with minimal or no risk of graft failure and acceptable GvHD rates.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Panepinto JA, Bonner M. Health-related quality of life in sickle cell disease: past, present, and future. Pediatr Blood Cancer. 2012;59:377–85.
Rees DC, Williams TN, Gladwin MT. Sickle-cell disease. Lancet. 2010;376:2018–31.
Weatherall DJ. The inherited diseases of hemoglobin are an emerging global health burden. Blood. 2010;115:4331–6.
Weatherall DJ. The challenge of haemoglobinopathies in resource-poor countries. Br J Haematol. 2011;154:736–44.
McGann PT. Sickle cell anemia: an underappreciated and unaddressed contributor to global childhood mortality. J Pediatr. 2014;165:18–22.
Piel FB, Hay SI, Gupta S, Weatherall DJ, Williams TN. Global burden of sickle cell anaemia in children under five, 2010–2050: modelling based on demographics, excess mortality, and interventions. PloS Med. 2013;10:e1001484.
Powars DR, Chan LS, Hiti A, Ramicone E, Johnson C. Outcome of sickle cell anemia: a 4-decade observational study of 1056 patients. Medicine. 2005;84:363–76.
Hulbert ML, McKinstry RC, Lacey JL, Moran CJ, Panepinto JA, Thompson AA, et al. Silent cerebral infarcts occur despite regular blood transfusion therapy after first strokes in children with sickle cell disease. Blood. 2011;117:772–9.
Johnson FL, Look AT, Gockerman J, Ruggiero MR, Dalla-Pozza L, Billings FT III. Bone-marrow transplantation in a patient with sickle-cell anemia. N Engl J Med. 1984;311:780–3.
Shenoy S. Hematopoietic stem cell transplantation for sickle cell disease: current practice and emerging trends. Hematol Am Soc Hematol Educ Program. 2011;1:273–9.
Jacobsohn DA, Duerst R, Tse W, Kletzel M. Reduced intensity haemopoietic stem-cell transplantation for treatment of non-malignant diseases in children. Lancet. 2004;364:156–62.
Iannone R, Casella JF, Fuchs EJ, Chen RA, Jones RJ, Woolfrey A, et al. Results of minimally toxic nonmyeloablative transplantation in patients with sickle cell anemia and beta thalassemia. Biol Blood Marrow Transpl. 2003;9:519–28.
Hsieh MM, Kang EM, Fitzhugh CD, Link MB, Bolan CD, Kurlander R, et al. Allogeneic hematopoietic stem-cell transplantation for sickle cell disease. N Engl J Med. 2009;361:2309–17.
Dew A, Collins D, Artz A, Rich E, Stock W, Swanson K, et al. Paucity of HLA identical unrelated donors for African-Americans with hematologic malignancies: the need for new donor options. Biol Blood Marrow Transpl. 2008;14:938–41.
Brodsky RA, Luznik L, Bolaños-Meade J, Leffell MS, Jones RJ, Fuchs EJ. Reduced intensity HLAhaploidentical BMT with post transplantation cyclophosphamide in nonmalignant hematologic diseases. Bone Marrow Transpl. 2008;42:523–7.
Luznik L, O’Donnell PV, Symons HJ, Chen AR, Leffell MS, Zahurak M, et al. HLA haploidentical bone marrow transplantation for hematologic malignancies using nonmyeloablative conditioning and high-dose, post-transplantation cyclophosphamide. Biol Blood Marrow Transpl. 2008;14:641–50.
Luznik L, Jalla S, Engstrom LW, Iannone R, Fuchs EJ. Durable engraftment of major histocompatibility complex-incompatible cells after nonmyeloablative conditioning with fludarabine, low-dose total body irradiation, and post-transplantation cyclophosphamide. Blood. 2001;98:3456–64.
Brunstein CG, Fuchs EJ, Carter SL, Karanes C, Costa LJ, et al. Alternative donor transplantation after reduced intensity conditioning: results of parallel phase 2 trials using partially HLA-mismatched related bone marrow or unrelated double umbilical cord blood grafts. Blood. 2011;118:282–8.
Bolanos-Meade J, Fuchs EJ, Luznik L, Lanzkron SM, Gamper CJ, Jones RJ, et al. HLA-haploidentical bone marrow ~ transplantation with posttransplant cyclophosphamide expands the donor pool for patients with sickle cell disease. Blood. 2012;120:4285–91.
Pawlowska AB, Cheng JC, Karras NA, Sun W, Wang LD, Bell AD, et al. HLA haploidentical stem cell transplant with pretransplant immunosuppression for patients with sickle cell disease. Biol Blood Marrow Transpl. 2018;24:185–9.
Frangoul H, Evans M, Isbell J, Bruce K, Domm J. Haploidentical hematopoietic stem cell transplant for patients with sickle cell disease using thiotepa, fludarabine, thymoglobulin, low-dose cyclophosphamide, 200 cGy TBI, and post-transplant cyclophosphamide. Bone Marrow Transpl. 2018;53:647–50.
De La Fuente J, Dhedin N, Koyama T, Bernaudin F, Kuentz M, Karnik L, et al. Haploidentical bone marrow transplantation with post-transplant cyclophosphamide plus thiotepa improves donor engraftment in patients with sickle cell anemia: Results of an international learning collaborative. Biol Blood Marrow Transpl. 2019;25:1197–209.
Bhat S, Ngangbam S, Iqbal W, Badiger S, Damodar S, Nataraj KS, et al. A novel pre-transplant immunosuppressive preparative regimen for myeloablative haploidentical and unrelated donor hematopoietic transplant in hemoglobinopathies: a safe and effective approach. Biol Blood Marrow Transpl. 2017;23:S18–S391.
Bernaudin F, Socie´ G, Kuentz M, Chevret S, Duval M, Bertrand Y, et al. Long-term results of related myeloablative stem-cell transplantation to cure sickle cell disease. Blood. 2007;110:2749–56.
Vermylen C. Hematopoietic stem cell transplantation in sickle cell disease. Blood Rev. 2003;17:163–6.
Powars D, Weidman JA, Odom-Maryon T, Niland JC, Johnson C. Sickle cell chronic lung disease: prior morbidity and the risk of pulmonary failure. Medicine. 1988;67:66–76.
Ciurea SO, Cao K, Fernandez-Vina M, Kongtim P, Malki MA, Fuchs E, et al. The European Society for Blood and Marrow Transplantation (EBMT) consensus guidelines for the detection and treatment of donor specific anti-HLA antibodies (DSA) in haploidentical hematopoietic cell transplantation. Bone Marrow Transpl. 2018;53:521–34.
Leonard A, Bonifacino A, Dominical VM, Conrey A, Coles W, Link M, et al. Bone marrow characterization in sickle cell disease: inflammation and stress erythropoiesis lead to suboptimal CD34 recovery compared to normal volunteer bone marrow. Blood. 2017;130:966.
Rutella S, Filippini P, Bertania V, Pira GL, Altomare L, Ceccarelli S, et al. Mobilization of healthy donors with plerixafor affects the cellular composition of T-cell receptor (TCR)-αβ/Cd19-depleted haploidentical stem cell graft. J Transl Med. 2014;12:240.
Locatelli F, Pagliara D. Allogeneic hematopoietic stem cell transplantation in children with sickle cell disease. Pediatr blood Cancer. 2012;59:372–6. https://doi.org/10.1002/pbc.24177
Vermylen C, Cornu G, Ferster A, Brichard B, Ninane J, Ferrant A, et al. Haematopoietic stem cell transplantation for sickle cell anaemia: the first 50 patients transplanted in Belgium. Bone Marrow Transpl. 1998;22:1–6. https://doi.org/10.1038/sj.bmt.1701291
Walters MC, Patience M, Leisenring W, Eckman JR, Scott JP, Mentzer WC, et al. Bone marrow transplantation for sickle cell disease. N Engl J Med. 1996;335:369–76. https://doi.org/10.1056/NEJM199608083350601
Anurathapan U, Hongeng S, Pakakasama S, Sirachainan N, Songdej D, Chuansumrit A, et al. Hematopoietic stem cell transplantation for homozygous beta-thalassemia and beta-thalassemia/ hemoglobin E patients from haploidentical donors. Bone Marrow Transpl. 2016;51:813–8.
Acknowledgements
We would like to acknowledge Ms Manju Joseph and the entire nursing team for the excellent execution of the protocol and state of art clinical care given to the patients, to Ms Himshikha Yadav who fulfilled the job of BMT coordinator to best of her capacity. Ms Bharti Sharma, Senior data analyst helped in maintenance and compilation of the entire data and also in statistical analysis.
Author information
Authors and Affiliations
Contributions
GK conceptualized the protocol and drafted the paper. SA, AB, AR compiled the data and did the statistical analysis. All the authors contributed, read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
Center for Bone Marrow Transplant and Cellular Therapy, Indraprastha Apollo Hospital, New Delhi, India.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kharya, G., Bakane, A., Agarwal, S. et al. Pre-transplant myeloid and immune suppression, upfront plerixafor mobilization and post-transplant cyclophosphamide: novel strategy for haploidentical transplant in sickle cell disease. Bone Marrow Transplant 56, 492–504 (2021). https://doi.org/10.1038/s41409-020-01054-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41409-020-01054-3