Summary:
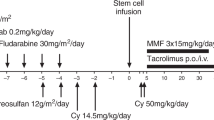
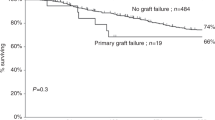
The feasibility of using lymphoablative rather than myeloablative conditioning for durable engraftment of allogeneic stem cells and subsequent cell therapy with donor lymphocytes was pioneered in the prefludarabine era in patients with resistant lymphoma and metastatic solid tumors. Between July 1995 and August 1996, 15 patients, five males and 10 females, median age 50 (range 20–57) years, were enrolled in a protocol that consisted of different doses of cyclophosphamide (Cy), 50 mg/kg/day for 1, 2, 3 or 4 consecutive days in parallel with a fixed dose of rabbit antithymocyte globulin (ATG) (Fresenius) 10 mg/kg/day for 4 consecutive days. All patients, except one treated with a single dose of Cy, achieved full tri-lineage engraftment and no late graft failure was observed. Only three patients suffered from grade III–IV graft-versus-host disease (GVHD). Three patients out of the 15 survived long term (follow-up >93 to >96 months). We concluded that lymphoablative conditioning with ATG and intermediate-to-high-dose Cy is well tolerated and can result in durable engraftment with acceptable GVHD in heavily pretreated patients with advanced malignancies. Hence, induction of tolerance to donor alloantigens by lymphoablative conditioning while avoiding myeloablative chemotherapy or radiation therapy may serve as a platform for subsequent cell therapy with donor lymphocytes.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Santos GW, Owens Jr AH . Allogeneic marrow transplants in cyclophosphamide treated mice. Transplant Proc 1969; 1: 44–46.
Storb R, Buckner CD, Dillingham LA, Thomas ED . Cyclophosphamide regimens in rhesus monkeys with and without marrow infusion. Cancer Res 1970; 30: 2195–2203.
Storb R, Epstein RB, Rudolph RH, Thomas ED . Allogeneic canine bone marrow transplantation following cyclophosphamide. Transplantation 1969; 7: 378–386.
Santos GW, Sensenbrenner LL, Burke PJ et al. Marrow transplantation in man following cyclophosphamide. Transplant Proc 1971; 3: 400–406.
Thomas ED, Buckner CD, Storb R et al. Aplastic anemia treated by marrow transplantation. Lancet 1972; 1: 284–289.
Storb R, Epstein RB, Rudolph RH, Thomas ED . The effect of prior transfusion on marrow grafts between histocompatible canine siblings. J Immunol 1970; 105: 627–633.
Storb R, Rudolph RH, Graham TC, Thomas ED . The influence of transfusions from unrelated donors upon marrow grafts between histocompatible canine siblings. J Immunol 1971; 107: 409–413.
Storb R, Weiden PL, Sullivan KM et al. Second marrow transplants in patients with aplastic anemia rejecting the first graft: use of a conditioning regimen including cyclophosphamide and antithymocyte globulin. Blood 1987; 70: 116–121.
Storb R, Etzioni R, Anasetti C et al. Cyclophosphamide combined with antithymocyte globulin in preparation for allogeneic marrow transplants in patients with aplastic anemia. Blood 1994; 84: 941–949.
Storb R, Blume KG, O'Donnell MR et al. Cyclophosphamide and antithymocyte globulin to condition patients with aplastic anemia for allogeneic marrow transplantation: the experience in four centers. Biol Blood Marrow Transplant 2001; 7: 39–44.
Kroger N, Zabelina T, Renges H et al. Long-term follow-up of allogeneic stem cell transplantation in patients with severe aplastic anemia after conditioning with cyclophosphamide plus antithymocyte globulin. Ann Hematol 2002; 81: 627–631.
Abdelkefi A, Ben Othman T, Ladeb S et al. Bone marrow transplantation for patients with acquired severe aplastic anemia using cyclophosphamide and antithymocyte globulin: the experience from a single center. Hematol J 2003; 4: 208–213.
Slavin S, Naparstek E, Nagler A et al. Allogeneic cell therapy for relapsed leukemia following bone marrow transplantation with donor peripheral blood lymphocytes. Exp Hematol 1995; 23: 1553–1562.
Kolb HJ, Mittermuller J, Clemm C et al. Donor leukocyte transfusions for treatment of recurrent chronic myelogenous leukemia in marrow transplant patients. Blood 1990; 76: 2462–2465.
Slavin S, Naparstek E, Nagler A et al. Allogeneic cell therapy with donor peripheral blood cells and recombinant human interleukin-2 to treat leukemia relapse post allogeneic bone marrow transplantation. Blood 1996; 87: 2195–2204.
Collins RH, Shpilber O, Drobyski WR et al. Donor leukocyte infusions in 140 patients with relapsed malignancy after allogeneic bone marrow transplantation. J Clin Oncol 1997; 15: 433–444.
Slavin S, Nagler A, Naparstek E et al. Non-myeloablative transplantation and cell therapy as an alternative to conventional bone marrow transplantation with lethal cytoreduction for the treatment of malignant and non-malignant hematologic diseases. Blood 1998; 91: 756–763.
Glucksberg H, Storb R, Fefer A et al. Clinical manifestations of graft-versus-host disease in human recipient of marrow from HLA-matched sibling donors. Transplantation 1974; 18: 295–304.
Atkinson K, Horowitz MM, Gale RP et al. Consensus among bone marrow transplanters for diagnosis, grading and treatment of chronic graft-versus-host disease. Committee of the International Bone Marrow Transplant Registry. Bone Marrow Transplant 1989; 4: 247–254.
Pugatsch T, Oppenheim A, Slavin S . Improved single-step PCR assay for sex identification post-allogeneic sex-mismatched BMT. Bone Marrow Transplant 1996; 17: 273–275.
Nakamura Y, Leppert O, O'Connel P et al. Variable number of tandem repeats (VNTR) markets for human gene mapping. Science 1987; 235: 1616–1622.
Porter DL, Connors JM, Van Deerlin VMD et al. Graft-versus-tumor induction with donor leukocyte infusions as primary therapy for patients with malignancies. J Clin Oncol 1999; 17: 1234–1243.
Ballen KK, Becker PS, Emmons RVB et al. Low-dose total body irradiation followed by allogeneic lymphocyte infusion may induce remission in patients with refractory hematologic malignancy. Blood 2002; 100: 442–450.
Slavin S . Immunotherapy of cancer with alloreactive lymphocytes. Lancet Oncology 2001; 2: 491–498.
Slavin S, Ackerstein A, Morecki S et al. Immunotherapy of relapsed resistant chronic myelogenous leukemia post allogeneic bone marrow transplantation with alloantigens pulsed donor lymphocytes. Bone Marrow Transplant 2001; 28: 795–798.
Slavin S, Strober S, Fuks Z, Kaplan HS . Long-term survival of skin allografts in mice treated with fractionated total lymphoid irradiation. Science 1976; 193: 1252–1254.
Slavin S, Strober S, Fuks Z, Kaplan HS . Use of total lymphoid irradiation in tissue transplantation in mice. Transplant Proc 1977; 9: 1001–1004.
Slavin S . Total lymphoid irradiation (TLI). Immunol Today 1987; 8: 88–92.
Childs R, Chernoff A, Contentin N et al. Regression of metastatic renal cell cancer following nonmyeloablative allogeneic peripheral blood stem cell transplantation. N Engl J Med 2000; 343: 750–758.
Raanani P, Dazzi F, Sohal J et al. The rate and kinetics of molecular response to donor leucocyte transfusions in chronic myeloid leukaemia patients treated for relapse after allogeneic bone marrow transplantation. Br J Haematol 1997; 99: 945–950.
Weiss L, Lubin I, Factorowich Y et al. Effective graft vs leukemia effects independent of graft vs host disease after T-cell depleted allogeneic bone marrow transplantation in a murine model of B cell leukemia/lymphoma. Role of cell therapy and rIL-2. J Immunol 1994; 153: 2562–2567.
Hahne M, Rimoldi D, Schröter M et al. Melanoma cell expression of Fas (Apo-1/CD95) ligand: implications for tumor immune escape. Science 1996; 274: 1363–1366.
Deeg HJ, Prentice R, Fritz TE et al. Increased incidence of malignant tumors in dogs after total body irradiation and marrow transplantation. Int J Radiat Oncol Biol Phys 1983; 9: 1505–1511.
Sanders JE, Hawley J, Levy W et al. Pregnancies following high-dose cyclophosphamide with or without high-dose busulfan or total-body irradiation and bone marrow transplantation. Blood 1996; 87: 3045–3052.
Eiermann TH, Lembrecht P, Zander AR . Monitoring anti-thymocyte globulin (ATG) in bone marrow recipients. Bone Marrow Transplant 1999; 23: 779–781.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bitan, M., Or, R., Shapira, M. et al. Nonmyeloablative stem cell transplantation using lymphoablative rather than myeloablative conditioning in the prefludarabine era by ATG and limiting doses of cyclophosphamide. Bone Marrow Transplant 35, 953–958 (2005). https://doi.org/10.1038/sj.bmt.1704936
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.bmt.1704936