Summary:
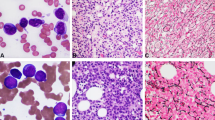
A young female patient in a second remission of acute lymphoblastic leukemia underwent bone marrow transplantation after total body irradiation and high-dose cytarabine from her HLA-matched brother. Following successful engraftment, mixed chimerism was seen 75 days post transplant. The karyotype contained numerous abnormalities in residual recipient cells. Chromosomes 1, 7, 13, and X were significantly more affected than other chromosomes. The high-frequency breakpoints identified were 1p22.2, 5q31.2, and 13q14.2. Some karyotypes specific for leukemia, such as t(9;22)(q34.1;q11.2) and t(8;21)(q22.2;q22.2), not seen with the original disease, were also present. As the frequency of aberrant chromosomes increased markedly with time, donor leukocytes were infused 14 months after BMT, which effectively eradicated the abnormal karyotypes.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Bhatia S, Ramsay NKC, Steinbuch M et al. Malignant neoplasms following bone marrow transplantation. Blood 1996; 87: 3633–3639.
Baker KS, DeFor TE, Burns LJ et al. New malignancies after blood or marrow stem-cell transplantation in children and adults: incidence and risk factors. J Clin Oncol 2003; 21: 1352–1358.
Neglia JP, Meadows AT, Robison LL et al. Second neoplasms after acute lymphoblastic leukemia in childhood. N Engl J Med 1991; 325: 1330–1336.
Pui C-H, Ribeiro RC, Hancock ML et al. Acute myeloid leukemia in children treated with epipodophyllotoxins for acute lymphoblastic leukemia. N Engl J Med 1991; 325: 1682–1687.
Taylor AMR, Metcalfe JA, Thick J, Mak Y-F . Leukemia and lymphoma in ataxia telangiectasia. Blood 1996; 87: 423–438.
Alter BP . Fanconi's anemia and malignancies. Am J Hematol 1996; 53: 99–110.
Milligan DW, De Elvira MCR, Kolb H-J et al. Secondary leukaemia and myelodysplasia after autografting for lymphoma: results from the EBMT. Br J Haematol 1999; 106: 1020–1026.
Pedersen-Bjergaard J, Pedersen M, Roulston D, Philip P . Different genetic pathways in leukemogenesis for patients presenting with therapy-related myelodysplasia and therapy-related acute myeloid leukemia. Blood 1995; 86: 3542–3552.
Mamuris Z, Gerbault-Seureau M, Prieur M et al. Chromosomal aberrations in lymphocytes of patients treated with melphalan. Int J Cancer 1989; 43: 80–86.
Barrios L, Miró R, Caballin MR et al. Cytogenetic effects of radiotherapy. Breakpoint distribution in induced chromosome aberrations. Cancer Genet Cytogenet 1989; 41: 61–70.
Socié G, Curtis RE, Deeg HJ et al. New malignant diseases after allogeneic marrow transplantatin for childhood acute leukemia. J Clin Oncol 2000; 18: 348–357.
Lin Y-W, Hamahata K, Watanabe K et al. Repetitious appearance and disappearance of different kinds of clonal cytogenetic abnormalities after allogeneic bone marrow transplantation. Int J Hematol 2001; 74: 86–89.
Imrie KR, Dubé I, Prince HM et al. New clonal karyotypic abnormalities acquired following autologous bone marrow transplantation for acute myeloid leukemia do not appear to confer an adverse prognosis. Bone Marrow Transplant 1998; 21: 395–399.
Baker MC, Lawler SD, Harris H et al. Radiation damage in patients treated by total-body irradiation, bone marrow grafting, and cyclosporin. Radiat Res 1986; 105: 413–424.
Przepiorka D, Ramberg R, Thomas ED et al. Host metaphases after chemoradiotherapy and allogeneic bone marrow transplantation for acute nonlymphoblastic leukemia. Leuk Res 1989; 13: 661–665.
Kano Y, Little JB . Site-specific chromosomal rearrangements induced in human diploid cells by x-irradiation. Cytogenet Cell Genet 1986; 41: 22–29.
Kamada N, Tanaka K . Cytogenetic studies of hematological disorders in atomic bomb surviviors. In: Ishihara T, Sasaki MS (eds). Radiation-Induced Chromosome Damage in Man. Alan R Liss, Inc.: New York, 1983, pp 455–474.
Ishihara T, Kumatori T . Cytogenetic follow-up studies in Japanese fishermen exposed to fallout radiation. In: Ishihara T, Sasaki MS (eds). Radiation-Induced Chromosome Damage in Man. Alan R Liss, Inc.: New York, 1983, pp 475–490.
Perot C, van den Akker J, Laporte JP et al. Multiple chromosome abnormalities in patients with acute leukemia after autologous bone marrow transplantation using total body irradiation and marrow purged with mafosfamide. Leukemia 1993; 7: 509–515.
Acknowledgements
We thank Dr Hiroshi Nishida for help with statistical analysis.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yoshihara, T., Hibi, S., Yamane, Y. et al. Numerous nonclonal chromosomal aberrations arising in residual recipient hematopoietic cells following allogeneic bone marrow transplantation. Bone Marrow Transplant 35, 587–589 (2005). https://doi.org/10.1038/sj.bmt.1704860
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.bmt.1704860
Keywords
This article is cited by
-
Therapy-related myeloid neoplasms of recipient origin after allogeneic hematopoietic stem cell transplantation for acute leukemia
International Journal of Hematology (2022)
-
Therapy-related myelodysplastic syndrome of recipient origin in a juvenile myelomonocytic leukemia patient 17 years after allogeneic BMT
Bone Marrow Transplantation (2011)
-
Allogeneic stem cell transplantation in children with acute lymphoblastic leukemia after isolated central nervous system relapse: our experiences and review of the literature
Bone Marrow Transplantation (2006)