Abstract
The hypothermia produced by 5-HT1A agonists had initially been claimed to be caused by the activation of cell body 5-HT1A autoreceptors resulting in decreased 5-HT transmission in laboratory animals. In order to address this issue in humans, 12 healthy volunteers underwent a dietary tryptophan depletion paradigm to decrease 5-HT availability, under double-blind conditions, during which body temperature was monitored following oral administration of the 5-HT1A agonist buspirone (30 mg). In addition, plasma prolactin and growth hormone evaluations, two responses that are mediated via the direct activation of postsynaptic 5-HT1A receptors, were determined. The hypothesis was that if responses are mediated by decreased transmission at postsynaptic 5-HT1A receptors, resulting from dampened 5-HT release as a consequence of 5-HT1A autoreceptors activation, then responses to the exogenous 5-HT1A agonist should be attenuated when 5-HT availability has been markedly decreased beforehand. Buspirone produced the same significant increase in prolactin and growth hormone in the tryptophan-depleted state as in the control condition. Similarly, the degree of hypothermia produced by buspirone was not significantly different in the two experimental conditions. In conclusion, these results strongly suggest that the hypothermia and the increases in prolactin and growth hormone produced by buspirone are attributable to the enhanced activation of postsynaptic 5-HT1A receptors, and not to a decrease in 5-HT transmission resulting from the activation of the 5-HT1A cell body autoreceptors on 5-HT neurons.
Similar content being viewed by others
Main
Serotonin (5-HT)1A receptors are located both on the cell body of 5-HT neurons and on postsynaptic neurons throughout the central nervous system. On 5-HT neurons, 5-HT1A receptors mediate a negative feedback influence on firing activity. When overactivated by an excess amount of 5-HT, or by an exogenous agonist, they slow down the pacemaker activity leading to a decreased firing rate of 5-HT neurons (Aghajanian and Lakoski 1984; Blier and de Montigny 1987). As 5-HT release is proportional to firing rate, the excess activation of these 5-HT1A autoreceptors generally results in a decrease of 5-HT release in projecting structures (see Rueter et al. 1997). The postsynaptic 5-HT1A receptors, particularly abundant in the limbic system, commonly exert an inhibitory function on neuronal activity as well. Therefore, the net effect of the systemic administration of a 5-HT1A receptor agonist on 5-HT1A signal transfer in postsynaptic areas represents a composite of decreased 5-HT release, resulting from 5-HT1A autoreceptor activation, and direct occupation of the postsynaptic 5-HT1A receptors.
Although it is well established that neuroendocrine responses, such as growth hormone and adenocorticotropic hormone release, produced by systemic administration of 5-HT1A agonists are mediated by postsynaptic 5-HT1A receptors (Van de Kar et al. 1985; Gilbert et al. 1988; Pan and Gilbert 1992), there has been controversy with respect to the hypothermia resulting from this pharmacological challenge. The very first study examining the pre- versus postsynaptic mediation of the hypothermia clearly indicated that this response was produced by postsynaptic 5-HT1A receptors (Hjörth 1985). Indeed, this initial study clearly showed that the hypothermic response to the prototypical 5-HT1A agonist 8-OH-DPAT was not attenuated, but even enhanced in rats pretreated daily for 3 days with the 5-HT synthesis inhibitor para-chlorophenylalanine (PCPA). Subsequently, identical results were obtained by another group of investigators using 2-day injections of a higher dose of PCPA (Goodwin et al. 1987). When their PCPA treatment was prolonged, however, for 14 days, despite producing its maximal effect on 5-HT levels after the first 3 days, the 8-OH-DPAT-induced hypothermia was dampened and the conclusion of a presynaptic mediation of this phenomenon was made. From then on, several studies carried out in laboratory animals and humans used the 5-HT1A-induced hypothermia to assess the responsiveness of the 5-HT1A autoreceptor in different disease states and following administration of various types of psychotropic drugs. Although the majority of studies (Hutson et al. 1987; O'Connell et al. 1992; Hillegaart 1991; Millan et al. 1993; Blier and Bouchard 1992; Stockmeier et al. 1992) now indicate that this response in rats results from the activation of postsynaptic 5-HT1A receptors, it is still claimed that it is presynaptically mediated in mice (Goodwin et al. 1985). In humans, this issue had not been addressed.
The present study was aimed at examining the 5-HT1A-mediated hypothermia in humans in a 5-HT depleted state. The hypothesis was that if it is mediated by a decreased 5-HT1A transmission at a postsynaptic site, resulting from dampened 5-HT release as a consequence of 5-HT1A autoreceptor activation, the hypothermic response to a 5-HT1A agonist should be attenuated or no longer present when 5-HT availability has been decreased beforehand. As PCPA is no longer used in humans, a dietary tryptophan depletion paradigm was utilized. This approach rapidly leads to significant decreases in brain 5-HT synthesis in humans (Nishizawa et al. 1997; Williams et al. 1999; Carpenter et al. 1998).
METHODS
Study Subjects
Fourteen healthy male volunteers were recruited through local newspaper advertisements. All potential research subjects underwent physical and psychiatric examinations (using the Structured Clinical Interview for DSM-III-R, nonpatient version; Spitzer et al. 1992). They had an electrocardiogram and routine laboratory tests, including serum sequential multiple analysis by computer, complete blood count, thyroid function tests, HIV test, hepatitis testing, urine analysis, and a urine toxicology screen for drugs of abuse. Only physically healthy subjects without a personal history of psychiatric illness or a family history of mood disorders or alcoholism in first-degree relatives were invited to participate. Subjects scoring 2 or more on at least 3 symptoms on the Hopkins Symptom checklist (HSCL-90) and more than 3 on the Beck Inventory for Depression were excluded. All subjects were medication free for a minimum of 2 weeks prior to testing, smoked less than 10 cigarettes per day, and ingested no more than the equivalent of 5 beers per week and 3 cups of coffee per day. A total of 12 subjects completed the study. Their average age was 29.3 ± 1.6 years (range 22–44). They weighed on average 75.5 ± 2.0 kg (range: 63.5–86.2), and their average height was 178.0 ± 1.7 cm (range 170.2–188.0). Their mean body mass index, calculated by dividing the weight in kg by the square of the height in meters, was 23.9 ± 0.7 (range: 20.3–27.7). This study was approved by the Research Ethics Board of the Department of Psychiatry of McGill University. Written informed consent was obtained from all subjects before enrollment in the study. They received $140 (CND) to compensate for loss of time related to their participation.
Procedure
The study was a double-blind crossover design. The day before testing, all subjects ate a low protein diet provided by the investigators and fasted from midnight before the experimental day (see Benkelfat et al. 1994). On the test day, subjects arrived at the laboratory at 8:30 A.M. and had blood samples drawn for the measurement of baseline plasma concentrations of tryptophan and baseline hormone levels using an IV catheter that was left in place with a heparin lock for the rest of the day. They then ingested one of two amino acid mixtures: (1) a 202-g nutritionally balanced control amino acid mixture containing 2.3 g of tryptophan, or (2) a similar mixture without tryptophan (Benkelfat et al. 1994). The drinks were prepared within a few minutes of oral administration by mixing the powdered amino acids with either: (1) 150 ml water, 45 ml chocolate syrup, and 0.6 g of sodium cyclamate; or (2) 180 ml of orange juice plus sodium cyclamate, according to the preference of the subjects. Because of the unpleasant taste of sulfur-containing amino acid methionine, cysteine, and arginine, these were encapsulated and administered separately.
Following ingestion of the amino acid mixture, the subjects remained awake in a room with affectively neutral videos and reading materials available to them. The 5-hour interval was chosen on the basis of data showing that, after ATD in humans, plasma tryptophan reaches its lowest level after about 5 h (Young et al. 1985), and CSF 5-hydroxyindole acetic acid levels are lowered at this time (Carpenter et al. 1998; Williams et al. 1999). Four hours after ingesting the amino acid mixture, the subjects lay down in a reclined bed in a comfortable position with the head elevated. They were not allowed to eat, sleep, or watch television until the procedure was completed. Following a 45-min rest period, baseline blood samples were obtained and oral temperature was measured. Immediately following collection of these data, subjects ingested three 10-mg tablets of buspirone (Buspar®) with a few sips of water. Additional blood samples were collected 60 and 120 min later. Oral temperature was measured every 15 min using a thermistor probe (Electro-Therm, Model TM99A), and digital readings were obtained at the end of a 2-min recording period.
At the end of each test day, before returning home, all subjects ate a high-protein snack and ingested a 1-g L-tryptophan tablet (Tryptan®). This tryptophan preparation, available only on prescription in Canada, has not been associated with any cases of eosinophilia myalgia syndrome (Wilkins 1990).
The samples for determination of growth hormone (GH) and prolactin were collected in ice-cold plastic tubes containing 10 μl/ml of 0.5 M Na2EDTA. They were centrifuged (20 min at 2,500 g at 4°C) within 1 h of sampling. Plasma was aliquoted into plastic tubes and stored at −70°C until time of assay. Plasma prolactin and GH levels were determined by standard immunoradiometric assays using commercially available kits (Immunocorp, Montréal, PQ; Nichols Institute Diagnostics, San Juan Capistrano, CA, USA). The inter- and intra-assay coefficients of variation were as follows: prolactin = 3.9 and 2.5%; growth hormone = 12.1 and 12.3%. Plasma tryptophan concentrations were measured by HPLC with fluometric detection as previously described (Benkelfat et al. 1994). At the end of the study, all measurements were carried out in one assay by a technician blind to the order and nature of drug treatments.
Statistical Analyses
All the results are expressed as means ± SEM. The data were submitted to analysis of covariance for repeated measures. There were no significant treatment X time interactions for any of the data sets, and therefore the two treatments were compared using a multivariate repeated analysis measure. All statistical analyses were performed with the Systat software package (Macintosh version, 1989). The level of statistical significance was taken as p < .05.
RESULTS
Tryptophan Levels
The total plasma tryptophan levels were nearly identical for the two test periods 24 h after a low protein diet. Five hours after ingestion of the drink containing tryptophan, total tryptophan was doubled whereas it was decreased by nearly 90% when the amino acid drink did not contain tryptophan. Two hours after the oral administration of buspirone, the total plasma tryptophan was back to the baseline value in the condition when the amino acid drink ingested contained tryptophan, but still decreased by more than 80% when the drink did not contain tryptophan (Figure 1 ). The difference in tryptophan levels between times 0 and 120 in the depleted state, however, did not quite reach statistical significance (t=1.91, p = .08).
Total plasma tryptophan (TRP) levels in healthy male volunteers prior to and following the injection of the amino acid mixture containing tryptophan (TRP balanced) or not (TRP depletion) at time –285 min. The subjects were on a low protein disk for 24 h prior to the first determination. At time 0, buspirone (30 mg) was taken orally. *Indicates a p < .05 using analysis of variance (ANOVA) for repeated measures when compared with the corresponding value obtained at baseline (time: −285 min.).
Neuroendocrine Assessments
Prolactin
During the initial depletion phase, prior to buspirone administration, there was a highly significant time effect [F1,22 = 20.6, p < .001]. There were, however, no significant order [F1,22=0.1, p = .8], subject [F11,12 = 0.8, p = .6] or time X treatment [F1,22 = 1.0, p = .3] effects. During the buspirone phase, there were no significant order [F2,21 = 0.2, p = .8], subject [F22,22 = 2.0, p = .06], or time X treatment effects [F2,20 = 0.40, p = .7]. In contrast, there was a highly significant time effect [F2,20 = 10.3, p < .001]. Although there was a significant elevation of prolactin levels 1 and 2 h after buspirone ingestion, the degree of enhancement was pronounced at time 60 [F1,22 = 14.1, p = .001], when usually a peak of prolactin is achieved following oral administration of a 5-HT1A agonist. These results indicate that, although prolactin levels fell during the day, they were then markedly enhanced by buspirone, independent of whether individuals were in a tryptophan depletion state or not (Figure 2 ).
Prolactin plasma levels in 12 healthy male volunteers prior to and following the ingestion of the amino acid mixture containing tryptophan (TRP balanced) or not (TRP depletion) at time –285 min. At time 0, buspirone (30 mg) was taken orally. *Indicates a p < .05 using ANOVA for repeated measures when compared with the corresponding value obtained at baseline (time: −285 min.) prior to buspirone administration, and afterward the baseline values were those obtained at time 0. There were no statistically significant differences between the values obtained in the TRP balanced and the TRP depletion conditions.
Growth Hormone
During the initial depletion phase, prior to buspirone administration, there was no significant order [F1,22 = 0.6, p = .4], subject [F1,11 = 1.2, p = .4], treatment [F1,22 = 2.1, p = .2], or time X treatment effects [F1,22 = 0.3, p = .6]. During the buspirone phase, there was a significant treatment effect [F2,21 = 3.6, p = 0.05], but no order [F1,21 = 0.5, p = .6] or subject effects [F2,21 = 0.2, p = .8]. These results indicate that there was no significant modification of growth hormone levels following the ingestion of amino acid drink, contrary to the results obtained with prolactin, and that buspirone significantly enhanced the levels of growth hormone regardless of whether the subjects were in a tryptophan depleted state or not (Figure 3 ).
Growth hormone plasma levels in 12 healthy male volunteers prior to and following the ingestion of the amino acid mixture containing tryptophan (TRP balanced) or not (TRP depletion) at time –285 min. At time 0, buspirone (30 mg) was taken orally. *Indicates a p < .05 using ANOVA for repeated measures when compared with the corresponding value obtained at time 0. There were no statistically significant differences between the values obtained in the TRP balanced and the TRP depletion conditions.
Body Temperature
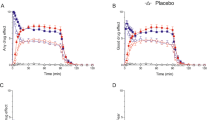
A total of 19 temperature deteminations were done, 10 prior to buspirone administration and 9 afterward. Body temperature had a tendency to rise following ingestion of the amino acid drink, which most likely reflected a diurnal change. Nevertheless, body temperatures remained stable in the 3 h preceding buspirone administration (Figure 4 ). Following buspirone administration, and taking the temperature 285 min after ingestion of the amino acid drinks as the baseline values, there was a significant time effect [F1,9 = 21.2, p = .001], but no subject [F11,9 = 0.3, p = .6], treatment [F1,9 = 1.5, p = .3], and treatment X time effects [F7,3 = 0.1, p = 1.0]. Consequently, buspirone exerted a significant hypothermic effect, and although there was a trend for a greater effect in the tryptophan-depleted state that would anyhow go against the hypothesis of a presynaptic mediation, this difference did not reach the predetermined level of statistical significance (Figure 5 ).
Evolution of oral temperature prior to and following the ingestion of the amino acid mixture containing tryptophan (TRP balanced) or not (TRP depletion) at time –285 min. The subjects were allowed to move around in the observation room until time –60 min. when they remained lying in bed. There were no statistically significant differences between the values obtained in the two conditions.
Hypothermic response obtained with the oral administration of buspirone (30 mg) in the same 12 male healthy volunteers tested 285 min. after the ingestion of an amino acid mixture containing tryptophan (TRP balanced) or not (TRP depletion). The baseline values were those illustrated at time 0 in Figure 4. The difference between the two curves was not statistically significant.
DISCUSSION
The results of the present study indicate that the hypothermia induced by the 5-HT1A agonist buspirone in humans was not attenuated in a 5-HT depleted state (Figure 5), just as for the prolactin and the growth hormone responses (Figures 2 and 3). These data, therefore, imply that all three responses are mediated by an increased activation of postsynaptic 5-HT1A receptors, and not by a decreased occupation of such receptors by endogenous 5-HT resulting from the agonistic activity of buspirone at the 5-HT1A autoreceptor.
Given the results obtained in the present experiments, the first possibility to consider is the adequacy of the tryptophan depletion paradigm to lower synaptic 5-HT availability. As depicted in Figure 1, total plasma tryptophan was decreased by about than 90% by the time the 5-HT1A agonist was administered. Although free plasma tryptophan, which is the important parameter for brain uptake, was not measured, previous studies clearly indicate that the two values are affected to the same extent with this dietary challenge (Young et al. 1985; Delgado et al. 1990). In addition, the following observations would support the notion that this tryptophan-dietary depletion paradigm is sufficient to exert a robust decrease in the synaptic availability of 5-HT in the human brain. This challenge has been shown to decrease tryptophan in the cerebrospinal fluid about as much as plasma tryptophan (Carpenter et al. 1998; Williams et al. 1999), but to a lesser extent than that of the metabolite 5-hydroxyindole acetic acid. Furthermore, 5-HT synthesis measured in humans, using α-[11C]methyL-tryptophan and positron emission tomography, is decreased approximately to the same extent as plasma tryptophan availability (Nishizawa et al. 1997). The possibility that the latter method is not merely a reflection of tryptophan plasma levels can tentatively be ruled out by the observations that different patient populations with similar plasma levels of tryptophan present different 5-HT synthesis rates (see Benkelfat et al. 1999; Leyton et al., 2001). Although this procedure does not trigger clear depressive symptoms in normal individuals, it is nevertheless sufficient to produce mild dysphoria, especially if they are subjected to a mild stress (Young et al., 1985; Leyton et al. 2000). Moreover, this paradigm produces, within the same time frame as that used in the present study, a resurgence of depressive symptoms in patients previously ameliorated by their antidepressant drug regimen (Delgado et al. 1990,1999; Bremner et al. 1997). Most importantly, it can produce a recurrence of depressive symptoms in remitted depressed patients who are no longer taking antidepressant drugs (Smith et al. 1997; Moreno et al. 2000; Spillmann et al. 2001). Consequently, the tryptophan depletion was likely sufficient to attenuate 5-HT transmission in the brain of subjects studied in the present experiments.
In rats, the hypothermia resulting from the systemic administration of 5-HT1A agonists is generally believed to be caused by the action of postsynaptic 5-HT1A receptor activation. The most compelling arguments in favor of this lore are the following. First, subacute 5-HT synthesis inhibition does not attenuate this response (Hjörth 1985; Hutson et al. 1987; O'Connell et al. 1992; Goodwin et al. 1985). Second, the lesioning of 5-HT neurons using the neurotoxin 5,7-dihydroxytryptamine also leaves unaffected the 5-HT1A-induced hypothermia (Millan et al. 1993). The observation, however, that intraraphe injection of 5-HT or of the 5-HT1A agonist 8-OH-DPAT produces hypothermia in rats may shed some doubt as to the postsynaptic mediation of this decrease in core temperature (Hillegaart 1991). It is nevertheless conceivable that if the solution injected diffused to the Sylvius aqueduct, located immediately above the dorsal raphe, a small amount of agonist could find its way to the third ventricle and reach to the hypothalamus located just rostrally to the dorsal raphe. Indeed, the above-mentioned experiments were carried out using 0.5 μL, whereas Bonvento et al. (1992) showed that only a volume of 0.1 μL of [3H]8-OH-DPAT injected in that region of the brainstem remained confined to the dorsal raphe. Furthermore, it was noted in the work of Higgins et al. (1988) that the amount of 8-OH-DPAT (injected directly into the dorsal raphe) necessary to produce hypothermia was 25 times greater than that necessary to trigger behavioral modifications.
Another line of evidence in favor of the hypothermia being mediated by postsynaptic 5-HT1A receptors stems from experiments examining the effects of repeated electroconvulsive shocks (ECS) on this parameter. This procedure has repeatedly been shown to attenuate the 8-OH-DPAT-induced hypothermia in rats (Goodwin et al. 1985; Blier and Bouchard 1992; Stockmeier et al. 1992). When the sensitivity of the 5-HT1A autoreceptor in the dorsal raphe, the presumed site of action of 5-HT1A agonists on core temperature, was assessed using direct microiontophoretic applications of 5-HT and 8-OH-DPAT, the response of 5-HT neurons was unaltered in ECS-treated rats (Blier and Bouchard 1992). In contrast, following repeated ECS, [3H]8-OH-PAT binding density was markedly decreased in rat hypothalamus (Stockmeier et al. 1992), a structure being a much more likely candidate to mediate the hypothermic response. Indeed, the neurons ultimately responsible for the decrease in body temperature are probably located in the medial preoptic region of the hypothalamus. This is based, in part, on the observations that increasing 5-HT in the medial preoptic region, either by stimulating the raphe nuclei or directly injecting 5-HT into this region, induces a decrease in core temperature in the rat (Lin et al. 1983). Nevertheless, the possibility that the 8-OH-DPAT-induced hypothermia could in fact be mediated by somatodendritic 5-HT1A autoreceptors of 5-HT neurons in the median raphe has to be considered. The medial preoptic region of the hypothalamus, which is located immediately adjacent to the third ventricle, is innervated, however, to only a small extent by fibers originating from the median raphe (Vertes and Martin 1988). It would therefore be unlikely that the administration of 8-OH-DPAT, which suppresses the firing activity of 5-HT neurons in the median and dorsal raphe (Sinton and Fallon 1988; Blier et al. 1990), and consequently reduces 5-HT release in the hypothalamus (Auerbach et al. 1989), would induce hypothermia by such a presynaptic mechanism.
In mice, the 5-HT1A receptor-induced hypothermia may appear to be mediated through 5-HT1A autoreceptor activation (Goodwin et al. 1985). Not all studies support this contention, however. For instance, Meller et al. (1992) did not observe any attenuation of the 5-HT1A receptor-induced hypothermia following three doses of PCPA to decrease 5-HT synthesis. Had the hypothermia been mediated in mice by the 5-HT1A autoreceptor, it should have been decreased by the 5-HT synthesis inhibitor. In addition, the dorsal raphe is generally considered to be endowed with a significant receptor reserve with respect to 5-HT1A autoreceptor function, which is not what these investigators have found. From a theoretical point of view, it is difficult to imagine that in rats and mice the same biological parameter could be mediated by diametrically opposed changes in 5-HT transmission, i.e., a presynaptic mediation leading to decreased 5-HT transmission and a postsynaptic one producing an increase in 5-HT transmission.
In conclusion, the bulk of the evidence indicates that the 5-HT1A receptor-induced hypothermia is mediated by postsynaptic 5-HT1A receptor activation in laboratory animals, and the present study, presumably carried out under adequate conditions, did not generate evidence to the contrary in humans. One may thus conclude that this physiological response in humans should not be used as a probe for the 5-HT1A autoreceptor. Such an erroneous use of this challenge may lead to false conclusions in disease states on receptor responsiveness, or to misleading information with respect to the effects of psychotropic drug treatments.
References
Aghajanian GK, Lakoski JM . (1984): Hyperpolarization of serotonergic neurons by serotonin and LSD: studies in brain slices showing increased K+ conductance. Brain Res 305: 181–185
Auerbach SB, Minzenberg MJ, Wilkinson LO . (1989): Extracellular serotonin and 5-hydroxyindoleacetic acid in hypothalamus of the unanesthetized rat measured by in vivo dialysis coupled to high-performance liquid chromatography with electrochemical detection: dialysate serotonin reflects neuronal release. Brain Res 499: 281–290
Benkelfat C, Ellenbogen MA, Dean P, Palmour M, Young SN . (1994): Mood-lowering effect of tryptophan depletion. Enhanced susceptibility in young men at genetic risk for major affective disorders. Arch Gen Psychiatry 51: 687–697
Benkelfat C, Young SN, Okazawa H, Leyton M, Diksic M . (1999): The validity of the PET/alpha-[11C]methyl-L-tryptophan method for measuring rates of serotonin synthesis in the human brain. Neuropsychopharmacology 21: 153–157
Blier P, de Montigny C . (1987): Modification of 5-HT neuron properties by sustained administration of the 5-HT1A agonist gepirone: electropysiological studies in the rat brain. Synapse 1: 470–480
Blier P, Serrano A, Scatton B . (1990): Differential responsiveness of the rat dorsal and median raphe 5-HT systems to 5-HT1 receptor agonists and p-chloroamphetamine. Synapse 5: 120–133
Blier P, Bouchard C . (1992): Effect of repeated electroconvulsive shocks on serotonergic neurons. Eur J Pharmacol 211: 365–373
Bonvento G, Scatton B, Claustre Y, Rouquier L . (1992): Effect of local injection of 8-OH-DPAT into the dorsal or median raphe nuclei on extracellular levels of serotonin in serotonergic projection areas in the rat brain. Neurosci Lett 137: 101–104
Bremner JD, Innis RB, Salomon RM, Staib LH, NG CK, Miller HL, Bronen RA, Krystal JH, Duncan J, Rich D, Price LH, Malison R, Dey H, Soufer R, Charney DS . (1997): Positron emission topography measurement of cerebral metabolic correlates tryptophan depletion-induced depressive relapse. Arch Gen Psychiatry 54: 364–374.
Carpenter LL, Anderson GM, Pelton GH, Gudin JA, Kirwin PD, Price LH, Heninger GR, McDougle CJ . (1998): Tryptophan depletion during continuous CSF sampling in healthy human subjects. Neuropsychopharmacology 19: 26–35
Delgado PL, Miller HL, Salomon RM, Licinio J, Krystal JH, Moreno FA, Heninger GR, Charney DS . (1999): Tryptophan-depletion challenge in depressed patients treated with desipramine or fluoxetine: implications for the role of serotonin in the mechanism of antidepressant action. Biol Psychiat 46: 212–220
Delgado PL, Charnery DS, Price LH, Aghanjanian GK, Landis H, Heninger GR . (1990): Serotonin function and the mechanism of antidepressant action. Reversal of antidepressant-induced remission by rapid depletion of plasma tryptophan. Arch Gen Psychiatry 47: 411–418
Gilbert F, Dourish CT, Brazell C . (1988): Relationship of increased food intake and plasma ACTH levels to 5-HT1A receptor activation in rats. Psychoneuroendocrinology 13: 471–478
Goodwin GM, De Souza RJ, Green AR . (1985): The pharmacology of the hypothermic response in mice to 8-hydroxy-2(di-n-propylamino) tetralin (8-OH-DPAT): a model of presynaptic 5-HT1 function. Neuropharmacology 24: 1187–1194
Goodwin GM, De Souza RJ, Green AR . (1987): Attenuation by electroconvulsive shock and antidepressant drugs of the 5-HT1A receptor mediated hypothermia and serotonin syndrome produced by 8-OH-DPAT in the rat. Psychopharmacology (Berl) 91: 500–505
Hillegaart V . (1991): Effects of local application of 5-HT and 8-OH-DPAT into the dorsal and median raphe nuclei on core temperature in the rat. Psychopharmacology (Berl) 103: 291–296
Higgins A, Bradbury AJ, Jones BJ, Oakley NR . (1988): Behavioural and biochemical consequences following activation of 5HT1-like and GABA receptors in the dorsal raphe nucleus of the rat. Neuropharmacology 27: 993–1001
Hjörth S . (1985): Hypothermia in the rat induced by the potent serotonergic agent 8-OH-DPAT. J Neural Transm 61: 131–135
Hutson PH, Donohoe TP, Curzon G . (1987): Hypothermia induced by the putative 5-HT1A agonists LY165163 and 8-OH-DPAT is not prevented by 5-HT depletion. Eur J Pharmacol 143: 221–228
Leyton M, Okazawa H, Diksic M, Paris J, Rosa P, Mzengeza S, Young SN, Blier P, Benkelfat C . (2001): Brain regional α[11C]Methyl-l-Tryptophan trapping in impulsive subjects with borderline personalitiy disorder. Am J Psychiatry 158: 775–782
Leyton M, Young SN, Pihl RD, Etezadi S, Lauze C, Blier P, Baker GB, Benkelfat C . (2000): Effects on mood of acute phenylalanine/tyrosine depletion in healthy women. Neuropsychopharmacology 22: 52–63
Lin MT, Wu JJ, Tsay BL, (1983): Serotonergic mechanisms in the hypothalamus mediate thermoregulatory responses in rats. Naunyn Schmiedeberg's Arch Pharmacol 322: 271–278
Meller E, Chalfin M, Bohmaker M . (1992): Serotonin 5-HT1A receptor-mediated hypothermia in mice: absence of spare receptors and rapid induction of tolerance. Pharmacol Biochem Behav 43: 405–411
Millan MJ, Rivet JM, Canton H, Marouille-Girardon S, Gobert A . (1993): Induction of hypothermia as a model of 5-hydroxytryptamine1A receptor-mediated activity in the rat: a pharmacological characterization of the actions of novel agonists and antagonists. J Pharmacol Exp Ther 264: 1364–1376
Moreno FA, Heninger GR, McGahuey CA, Delgado PL . (2000): Tryptophan depletion and risk of depression relapse: a prospective study of tryptophan depletion as a potential predictor of depressive episodes. Biol Psychiat 48: 327–329
Nishizawa S, Benkelfat C, Young SN, Leyton M, Mzengeza S, de Montigny C, Blier P, Diksic M . (1997): Differences between males and females in rates of serotonin synthesis in human brain. Proc Natl Acad Sci USA 94: 5308–5313
O'Connell MT, Sarna G, Curzon G . (1992): Evidence for postsynaptic mediation of the hypothermic effect of 5-HT1A receptor activation. Br J Pharmacol 106: 603–609
Pan LH, Gilbert F . (1992): Activation of 5-HT1A receptor subtype in the paraventricular nuclei of the hypothalamus induces CRH and ACTH release in the rat. Neuroendocrinology 56: 797–802
Rueter LE, Fornal CA, Jacobs BL . (1997): A critical review of 5-HT brain microdialysis and behavior. Rev Neurosci 8: 117–137
Smith KA, Fairburn CG, Cowen PJ . (1997): Relapse of depression after rapid depletion of tryptophan. Lancet 349 (9056): 915–919
Sinton CM, Fallon SL . (1988): Electrophysiological evidence for a functional differentiation between subtypes of the 5-HT1 receptor. Eur J Pharmacol 157: 173–181
Spillmann MK, Van der Does AJ, Rankin MA, Vuolo RD, Alpert JE, Nierenberg AA, Rosenbaum JF, Hayden D, Schoenfeld D, Fava M . (2001): Tryptophan depletion in SSRI-recovered depressed outpatients. Psychopharmacology (Berl) 155: 123–127
Spitzer RL, Williams JBW, Gibbon M, First MB . (1992): The structured clinical interview for DSM-III-R (SCID), I: History, rationale, and description. Arch Gen Psychiatry 49: 624–629
Stockmeier CA, Wingenfeld P, Gudelsky GA . (1992): Effects of repeated electroconvulsive shock on serotonin1A receptor binding and receptor-mediated hypothermia in the rat. Neuropharmacol 31: 1089–1094
Van de Kar LD, Karteszi M, Bethea CL, Ganong W . (1985): Serotonergic stimulation of prolactin and corticosterone secretion is mediated by different pathways from the mediobasal hypothalamus. Neuroendocrinology 41: 380–384
Vertes RP, Martin GF . (1988): Autoradiographic analysis of ascending projections from the pontine and mesencephalic reticular formation and the median raphe nucleus in the rat. J Comp Neurol 275: 511–541
Wilkins K, Wigle D . (1990): Eosinophilia-myalgia syndrome. Can MED ASSN J 142: 1265–1266
Williams WA, Shoaf SE, Hommer D, Rawlings R, Linnoila M . (1999): Effects of acute typtophan depletion on plasma and cerebrospinal fluid tryptophan and 5-hydroxyindoleacetic acid in normal volunteers. J Neurochem 72: 1641–1647
Young SN, Smith SE, Pihl RO, Ervin FR . (1985): Tryptophan depletion causes a rapid lowering of mood in normal males. Psychopharmacology (Berl) 87: 173–177
Acknowledgements
This work was supported in part by the Medical Research Council of Canada (MRC) grants MT-11014 and MT-15005, the Fonds de la Recherche en Santé du Québec, and from salary support from the University of Florida to PB.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Blier, P., Seletti, B., Gilbert, F. et al. Serotonin 1A Receptor Activation and Hypothermia in Humans: Lack of Evidence for a Presynaptic Mediation. Neuropsychopharmacol 27, 301–308 (2002). https://doi.org/10.1016/S0893-133X(02)00318-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1016/S0893-133X(02)00318-4
Keywords
This article is cited by
-
Blunted 5-HT1A receptor-mediated responses and antidepressant-like behavior in mice lacking the GABAB1a but not GABAB1b subunit isoforms
Psychopharmacology (2017)
-
Functional Interactions between 5-HT1A and 5-HT2A Receptors in the Brain
Neuroscience and Behavioral Physiology (2016)
-
Douleur et thermorégulation. La thermorégulation chez l’animal
Douleur et Analgésie (2015)
-
Serotonin and molecular neuroimaging in humans using PET
Amino Acids (2012)
-
Adaptations in pre- and postsynaptic 5-HT1A receptor function and cocaine supersensitivity in serotonin transporter knockout rats
Psychopharmacology (2008)