Abstract
Recent studies have found that acute morphine administration increases serotonin (5-HT) transmission within the nucleus accumbens and other forebrain regions. In contrast, 5-HT transmission is depressed during withdrawal from chronic morphine. We show that pharmacological agents that increase brain 5-HT levels (fluoxetine or 5-hydoxytryptophan, 5-HTP) abolish the preference of chronically morphine-treated, withdrawn rats for a morphine-associated environment. Similar results were seen when fluoxetine was microinjected into the nucleus accumbens. Conversely, rats given morphine acutely showed an enhanced preference for a morphine-associated environment when pretreated with these agents. Fluoxetine also decreased the heightened anxiety found in morphine withdrawn rats. The results of our study indicate that drugs that augment 5-HT levels may reduce the desire for morphine during withdrawal.
Similar content being viewed by others
Main
In addition to gross neurological and somatic signs the abstinence syndrome associated with opiate dependence involves important subjective responses including anxiety, anhedonia, depression and drug craving (Jaffe 1990). The control and elimination of the subjective symptoms, which linger long after the physical symptoms of opiate withdrawal have dissipated, has been a major obstacle to the successful treatment of opiate dependence (Childress et al. 1994). Understanding the alterations in brain chemistry that occur during abstinence from opiate abuse may lead to improved treatments for these symptoms, and greater success in treating opiate addiction.
Acute systemic administration of morphine enhances brain 5-HT synthesis (Garcia-Sevilla et al. 1980) and tryptophan hydroxylase activity (Boadle-Biber et al. 1987). Acute morphine also enhances the turnover and release of 5-HT and dopamine (DA) in widespread areas of the forebrain (Spampinato et al. 1985; Tao and Auerbach 1994). The effect of morphine on 5-HT release appears to be mediated by activation of dorsal raphe neurons (Spampinato et al. 1985; Tao and Auerbach 1994) and may reflect disinhibition caused by direct inhibition of GABA transmission. A similar mechanism has also been proposed for the excitatory effects of opiates on DA neurons in the ventral tegmental area (Bloom et al. 1972; Bonci and Williams 1997).
Neurons in the nucleus accumbens have been shown to be critically involved in the reinforcing properties of opiates (Dworkin et al. 1988; Pettit et al. 1984; Zito et al. 1985). Although morphine administration enhances 5-HT and DA levels within the accumbens (Spampinato et al. 1985; Tao and Auerbach 1995; Wise et al. 1995), the transmitter systems involved in opiate reward are not decisively known. It has been shown that chemical lesions of DA systems or blockade with dopamine antagonists do not affect opiate self-administration (Ettenberg et al. 1982; Gerrits and Van Ree 1996; Pettit et al. 1984) or morphine place preference in animals (Mackey and van der Kooy 1985; however, also see Bozarth and Wise 1981; Smith et al. 1985; Spyraki et al. 1983). On the other hand, chemical lesions of 5-HT terminals in the accumbens impair morphine self-administration without affecting responding for food or water (Smith et al. 1987), and also block the acquisition of morphine conditioned place preference (CPP) (Spyraki et al. 1988).
Withdrawal from opiates decreases both dopamine and 5-HT transmission in the brain. A single dose of morphine induces measurable reductions in both 5-HT and DA levels at 24 hours post-injection (Antkiewicz-Michaluk et al. 1995). Withdrawal from longer chronic opiate treatments produces profound decreases in both dopamine (Acquas et al. 1991; Pothos et al. 1991; Rossetti et al. 1992) and 5-HT transmission (Ahtee 1980; Tao et al. 1998). Reduced 5-HT levels have been linked to both depression (Maes and Meltzer 1995) and compulsive behavior (Dolberg et al. 1996), two disorders that have recently been proposed to play a role in addictive behaviors (Childress et al. 1994; Koob et al. 1998; Markou et al. 1998; O'Brien et al. 1998). Thus, withdrawal-induced reductions in monoamine levels could be responsible for somatic and well as subjective symptoms of opiate withdrawal. Previously, we found that dopamine transmission in the nucleus accumbens regulated somatic signs of opiate withdrawal (Harris and Aston-Jones 1994).
Here we examined the role of 5-HT transmission in subjective signs of opiate withdrawal. In the current series of experiments, we used a conditioned place preference (CPP) paradigm to test the possibility that decreased 5-HT levels during morphine withdrawal may be involved in the withdrawal-enhanced preference for a morphine environment. We hypothesize that after the physical signs of opiate withdrawal have dissipated decreased 5-HT may be responsible for some of the subjective signs of opiate withdrawal that persist. We also propose that the subjective signs of opiate withdrawal in rats could be expressed as an enhanced place preference for morphine and/or increased anxiety. We tested whether 5-HT enhancing agents might decrease the preference for morphine in withdrawing animals using CPP. In the CPP paradigm, drug preference is measured by the amount of time an animal spends in an environment that has been associated with morphine. Environmental drug cues have been shown to be potent stimulators of opiate craving in humans (Ehrman et al. 1992) and CPP models in animals the cue-elicited conditioning that motivates drug-taking behavior (Bardo et al. 1995; Tzschentke 1998). Because CPP training elicits an operant approach response even in the absence of drug, animals can be tested in a morphine-free state without the problems associated with extinction as might occur in a self-administration paradigm when morphine is not present at testing. Furthermore, CPP allows us to address the role of environmental cues associated with morphine withdrawal.
We also tested whether enhancing 5-HT levels in morphine-withdrawn animals would reduce the anxiety associated with morphine withdrawal (Harris and Aston-Jones 1993). For this, we used the conditioned defensive burying paradigm to measure the anxiety associated with drug withdrawal because of its speed, simplicity and reliability (Treit 1985) . This task measures species-specific defensive behavior and, unlike drug discrimination or conflict paradigms, does not require extensive behavioral training of the animals. Furthermore, in contrast to tasks such as the elevated plus maze or the light-dark box, this task contains both active instinctual and passive learning components that can be separately measured.
MATERIALS AND METHODS
Subjects
Male Sprague-Dawley rats (200-250 g) from Harlan (Indianapolis, IN) were used in all experiments. Rats were group housed in accordance with NIH guidelines on a 12-h light/dark cycle with food and water available ad libitum. All animal procedures were approved by the Institutional Animal Care and Use Committees of the Philadelphia Veterans Administration Medical Center and the University of Pennsylvania.
Drugs
Morphine pellets and morphine sulfate powder were provided by the National Institute on Drug Abuse. Fluoxetine was purchased from Tocris Cookson Inc (St Louis, MO) and 5-HTP was purchased from RBI (Natick, MA). Both drugs were dissolved in sterile distilled water. Morphine sulfate was dissolved in sterile saline. All vehicle injections consisted of sterile distilled water, and all drugs were administered via intraperitonal injection.
Chronic Drug Treatment
Rats under halothane anesthesia were implanted subcutaneusly with two 75 mg morphine tablets. Previous studies have shown that morphine pellets are a reliable way to induce physical dependence (Gold et al. 1994; Yoburn et al. 1985). The signs of physical dependence begin to wane around day 14 as the morphine pellets dissolve (Gold et al. 1994). Deprivation withdrawal (a model of abstinence) was induced by removing the pellets after 14 days.
Conditioned Place Preference Procedure
Testing occurred in a Plexiglas apparatus consisting of two distinct compartments separated by a tunnel. One compartment had a grid floor with black walls, and the second compartment had a mesh floor with black and white stripes on the walls. Each compartment was equipped with photocells to automatically record time in each compartment (MED Associates). On the first day (Day 14 of chronic morphine treatment), the rats were allowed to freely explore all of the apparatus for 15 min, and the amount of time spent in each compartment was recorded. None of the animals had an initial bias for either compartment and were randomly assigned to one compartment for morphine conditioning in a balanced design. Conditioning began five days later after all somatic signs of opiate withdrawal in the chronic morphine group had dissipated. On each of the next three days, rats were injected with either saline or morphine (20 mg/kg or 10 mg/kg ip in chronic morphine animals, and 10 mg/kg ip for the acute morphine group) in the morning and afternoon. Rats were confined to one side of the box after each injection by means of an opaque Plexiglas divider for 40 min. Morphine and saline treatments were alternated in morning and afternoon sessions for each animal, so that rats given morphine in the morning were given saline in the opposite compartment in the afternoon, and on subsequent days received saline in the morning, and morphine in the afternoon. One week following conditioning, acute and chronic morphine animals were pretreated with either vehicle, fluoxetine (10 mg/kg or 3.5 mg/kg) or 5-HTP (20 mg/kg), and 20 min later given free access to the apparatus for 15 min. The amount of time spent in each compartment was recorded. As an acute conditioning control, an additional group of six animals was given injections of saline in both compartments and morphine injections (10 mg/kg) in the home cage.
In a separate set of experiments, chronically morphine-treated withdrawn rats were given one-sided conditioning. Rats in these experiments were treated the same as above, except that one compartment was paired with morphine and the other compartment was only experienced on the preconditioning and test days. In this case the non-paired compartment was relatively novel to the animals and not associated with opiate withdrawal. Fifty-three animals were in the acute morphine groups and fifty-two animals were in the chronic morphine groups.
Intracerebral Injections
Under sodium pentobarbital anesthesia rats were implanted with bilateral chronic indwelling guide cannulae (26 gauge) aimed 2 mm above the nucleus accumbens (AP +1.6, ML 3.0, DV 6.3). Cannulae were angled 15° (dorsolateral to ventromedial) to avoid penetrating and allowing injectate to diffuse into the ventricle. The tips of inner injection cannulae (30 gauge) extended 2 mm below the guide cannulae into the injection sites. Control injections were made into the caudate nucleus (AP +1.6, ML 3.0, DV 6.1) in separate animals, 2 mm above accumbens injections. Animals were given one week to recover from surgery before receiving chronic drug treatments. Microinjections (0.5 μl volume on each side over 5 min) of fluoxetine (30 μM) or vehicle (artificial cerebral spinal fluid) were made 20 min prior to the final preference test. Animals were habituated to the procedure by mock injections (injection cannulae did not extend into the injection sites and no fluid was injected) given prior to each conditioning day. Twenty-three animals were included in the intra-accumbal groups, with group n = 5-6. Eleven animals had dorsal striatal injections.
Conditioned Defensive Burying Paradigm
As has been described previously for this task (Harris and Aston-Jones 1993; Treit et al. 1981), individual rats were placed in a chamber with an electrified prod inserted through a hole in the chamber wall. Electric current was supplied by a constant current source (MED Associates, 8 mA in amplitude) through two uninsulated silver wires wrapped around the prod. While exploring the prod, rats received one shock, and subsequently buried the prod using bedding material from the floor of the chamber. Burying time was measured for 15 min. Enhanced anxiety was evidenced by significantly shorter latencies to begin burying, as well as by an increase in burying duration, relative to saline-treated animals. Previous studies have validated this procedure to measure anxiety as evidenced by response to classical anxiolytic drugs (Treit 1985; Treit et al. 1981). Chronic and acute morphine animals pretreated with vehicle or fluoxetine were tested in the burying paradigm within 15 min of finishing the preference test. A third group consisted of chronic saline animals treated with vehicle.
5-HTP-Induced Head-Shake Behavior
Six animals from the vehicle-treated chronic morphine group and six from the vehicle-treated acute morphine group were administered 5-HTP (50 mg/kg) 30 min after the preference test. Head shake behavior as described previously (Lucki et al. 1984) consisted of a rapid rhythmic shaking of the head in a radial motion. The total number of head shakes during a 70-min period after drug administration were recorded. One-half of the animals were scored by a blind independent observer. There were no differences between the scores from the two observers.
Histology and Data Analysis
Animals from the intracerebral injection groups were killed with an overdose of sodium pentobarbital. Injections of pontine sky blue dye (0.5 μl) were made at injection sites to allow subsequent histological confirmation. Brains were removed and frozen with methylbutane and sectioned (40 μm). Sections were stained with neutral red to determine the exact location of the injection site. Only animals with injection sites located in the shell region of the nucleus accumbens or dorsal striatum (for the control injection group) were included in the data analysis. Place conditioning data were analyzed by calculating the time spent in the morphine-paired chamber minus the time spent in the saline-paired (or novel) chamber. The resulting difference score was compared between groups. In addition, a within-group measurement of conditioned place preference was assessed by comparing the difference in time spent in the morphine- and saline-paired side (or novel) preconditoning vs. postconditioning. Behavioral data were analyzed using a one-way or two-way analysis of variance. Where necessary post hoc analysis was performed with a Newman-Keuls test.
RESULTS
Place Conditioning
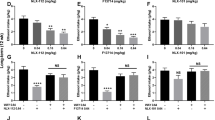
Our paradigm produced a significant preference for the morphine-paired environment in both acutely and chronically treated rats (p < .01; F(1,18) = 26.65 for acute, and F(1,15) = 27.51 for chronic animals). The acute control group given saline injections paired with both compartments and morphine in the home cage did not show a significant place preference (difference score = 25.8 ± 5.4 seconds, p > .53). A significantly enhanced preference for the morphine-paired environment was seen in rats given prior chronic morphine treatment (relative to the acute morphine group) (Figure 1A; F(1,13) = 13.14, p < .01). This enhanced preference was seen in the chronic morphine rats regardless of whether they were conditioned using the 10 mg/kg or 20 mg/kg dose of morphine (F(2,18) = 6.3, p < .01, see Table 1) The acute group was found to be significantly different from both chronic groups (p < .05), but the two chronic groups did not significantly differ from each other (p < .16).
Preference scores for the morphine-paired environment expressed as the time spent in the morphine-paired side minus the time spent in the saline-paired side on the test day. Groups were pretreated on the test day with vehicle, systemic fluoxetine (A, 3.5 and 10 mg/kg, ip), or systemic 5-HTP (B, 20 mg/kg, ip). Fluoxetine given systemically (A) significantly reduced the preference for the morphine-paired environment in the chronic morphine animals at the 3.5 mg/kg dose and abolished preference at the 10 mg/kg dose. Fluoxetine at both doses significantly enhanced preference in the acute morphine groups (A). Similarly, 5-HTP (B) significantly reduced the preference for the morphine-paired environment in the chronic morphine treated animals but enhanced preference in acute morphine animals. The symbol * indicates a significant difference between vehicle and drug groups (p < .05)
When animals from the chronic morphine group with two-sided conditioning were given systemic injections of fluoxetine on the test day, the preference for the morphine environment was reduced (3.5 mg/kg dose, p < .05) or eliminated (10 mg/kg dose, p < .01) (F(2,15) = 10.13, p < .01). Similar results were seen following the systemic administration of the 5-HT precursor 5-HTP on the test day (Figure 1B, F(1,10) = 93.05, p < .01). To tell whether fluoxetine decreased an aversion to the saline-paired environment in which morphine dependent animals might have experienced withdrawal, we also used a one-sided conditioning procedure. Fluoxetine (10 mg/kg) was also found to abolished the preference for the morphine-paired environment (Figure 2A) when administered to chronic morphine rats given one-sided conditioning (F(1,14) = 16.22, p < .01). These experiments also revealed that both the one-sided (Figure 1A) and two-sided (Figure 2A) conditioning procedures yielded similar levels of preference for the morphine-paired environment.
Preference scores for the morphine-paired environment expressed as the time spent in the morphine-paired side minus the time spent in the novel (A) or saline-paired side on the test day (B, C). Groups were pretreated on the test day with vehicle or systemic fluoxetine (A, 10 mg/kg, ip in chronic morphine animals given one-sided conditioning), intracranial fluoxetine (B, 30 μM microinjected into the accumbens of chronic morphine animals) or morphine (C, 10 mg/kg in acute morphine animals). Fluoxetine given systemically (A) significantly reduced the preference for the morphine-paired environment in the chronic morphine animals given one-sided conditioning. Micro-injections of fluoxetine (B) into the shell region of the nucleus accumbens of chronic morphine treated rats significantly decreased the preference for the morphine-paired environment. Morphine (C) given on the test day significantly enhanced preference in acute morphine animals. The symbol * indicates a significant difference between control and drug groups (p < .01)
Similar experiments were performed with intra-accumbens microinjections of fluoxetine or vehicle to test whether the nucleus accumbens was involved in the ability of 5-HT enhancing agents to decrease morphine preference in the chronic morphine group. Fluoxetine (30 μM) microinjected into the accumbal shell on the test day blocked the preference for the morphine-associated environment in the chronic morphine rats during both two-sided and one-sided conditioning (F(1,9) = 13.3, p < .01 for two-sided conditioning, data shown in Figure 2B and F(1,10) = 12.9, p < .01 for one-sided conditioning, the means were: Veh = 249 ± 69 and Flu = −4.0 ± 18). These results are similar to the findings obtained above with systemic fluoxetine or 5-HTP treatment. In contrast, fluoxetine had no effect on preference in such animals when injected dorsal to the accumbens along the cannula tract, in the caudate nucleus (F(1,8) = 0.10, p < .75, means Veh = 242 ± 44 Flu = 214 ± 50).
Unlike the effects seen in the chronic morphine animals, similar pretreatments with fluoxetine on the test day in rats acutely treated with morphine caused a significant enhancement in the preference for the morphine-paired environment at both the 3.5 (p < .01) and 10 mg/kg (p < .05) doses (Figure 1A F(2,20) = 11.2, p < .01). A similar enhancement was seen with 5-HTP administration (Figure 1B, F(1,10) = 47.47, p < .01). For the acute morphine-treated rats the increase in brain 5-HT levels caused by fluoxetine or 5-HTP may mimic some of the stimulus cueing properties of morphine, leading the animals to spend more time in the morphine-associated environment. To test this hypothesis, we pretreated acute morphine animals with morphine on the test day (10 mg/kg). These rats also showed an enhanced preference for the morphine environment (Figure 2C, F(1,10) = 68.4, p < .01), resembling the results found for such animals pretreated with fluoxetine or 5-HTP. The exact number of seconds each group in each of the drug conditions spent in the paired chambers can be seen in Table 1.
Head Shake Response
To test the idea that brain 5-HT systems were altered in the chronically morphine treated rats relative to the acute morphine rats, we injected animals with 5-HTP (50 mg/kg) and measured head shake behavior, an indirect measure of brain 5-HT function (Bedard and Pycock 1977; Darmani et al. 1997), for 1 hr. Acutely treated morphine rats showed significantly more head shakes than chronically treated morphine rats (19.2 ± 2.7 vs. 5.1 ± 0.36, F(1,10) = 25.7, p < .01). The number of head-shake responses seen in the acute group was similar to what was seen when the same dose of 5-HTP was given to drug-naive rats (18.5 ± 1.7, n = 4). Together, these results indicate that 5-HT transmission was depressed in the chronic morphine rats at the time of testing.
Conditioned Burying Response
Chronic morphine treatment significantly decreased the latency to begin burying and significantly increased the duration of burying in vehicle-pretreated rats even when tested one week after the last morphine injection (F(4,31) = 26.59, p < .01; the chronic morphine group was different from all other groups, p < .01). Fluoxetine pretreatment in the chronic morphine group increased the latency to begin burying and produced burying behavior similar to that seen in drug naive animals (Figure 3A). Fluoxetine also significantly reduced the enhanced amount of time that the morphine withdrawn rats buried (Figure 3b). No changes were seen with burying behavior in the acute morphine animals given fluoxetine.
Chronic morphine rats exhibited increased anxiety in a conditioned defensive burying paradigm. This figure shows the burying time latency (A, the time to begin burying after the shock) and duration (B, time spent burying) for chronic morphine rats pretreated with vehicle, chronic morphine rats pretreated with fluoxetine, and chronic saline rats pretreated with vehicle. Enhanced anxiety in chronic morphine treated rats was evidenced by significantly shorter latencies to begin burying as well as a significant increase in burying duration relative to chronic saline-treated animals. The withdrawal-induced increase in anxiety evidenced by burying behavior was blocked by pre-treatment with fluoxetine (10 mg/kg). The symbol * indicates a significant difference between chronic/vehicle and all other groups (p < .01)
DISCUSSION
The present results indicate that administration of either the 5-HT reuptake inhibitor, fluoxetine, or the 5-HT precursor, 5-HTP, strongly attenuated the preference of morphine-withdrawn subjects for a morphine-paired environment. This effect was specific for rats given chronic morphine, and did not occur in acutely treated morphine rats. In contrast to the effects seen in the chronic morphine treated rats, the acute morphine rats showed an augmented preference for the morphine environment when pretreated with the 5-HT enhancing drugs. Direct application of fluoxetine into the nucleus accumbens also abolished the preference for the morphine-associated environment in the chronically treated withdrawn animals, indicating that the accumbens is the likely site of action for the effect of fluoxetine. The decrease in preference seen in the chronic morphine rats following the administration of the 5-HT enhancing drugs occurred at a time when 5-HT transmission was reduced, as measured by the 5-HTP-induced head shake response. Fluoxetine was also shown to be effective in reducing anxiety in the chronically morphine-treated, withdrawn rats as measured in a defensive burying paradigm.
The fact that fluoxetine and 5-HTP were both effective in abolishing the preference for the morphine-associated environment in morphine-withdrawn rats indicates that a 5-HT mechanism is involved. Both drugs have been shown to substantially enhance forebrain 5-HT levels for many hours when given in the doses used in this experiment (Guan and McBride 1988; Gudelsky and Nash 1996; Perry and Fuller 1993; Rutter and Auerbach 1993). Fluoxetine blocks 5-HT reuptake to enhance 5-HT levels (Fuller et al. 1991) and 5-HTP is a 5-HT precursor and is decarboxylated in the brain to form 5-HT. As these two compounds enhance 5-HT levels by different mechanisms, it is unlikely that their similarities in effects result from nonspecific actions.
Previous studies have shown that 5-HT enhancing agents reduce the self-administration of opiates (Rockman et al. 1980; Wang et al. 1995). In addition, two research groups have reported that fluoxetine substantially reduces heroin craving in human addicts (Gerra et al. 1995; Maremmani et al. 1992). These studies, together with the current findings, indicate that enhancing 5-HT transmission in opiate experienced subjects may decrease the desire for morphine.
The enhancement of deficient 5-HT function in the withdrawn animals by fluoxetine and 5-HTP apparently reduced their motivation to spend time in the morphine-paired environment. The 10 mg/kg dose of fluoxetine and the 20 mg/kg dose of 5-HTP completely abolished the preference for the morphine environment in the chronically treated animals. The lower 3.5 mg/kg dose of fluoxetine had a smaller, although significant effect, and did not reduce preference below that of the acute group. The complete suppression of preference by the higher doses of the 5-HT enhancing agents does not appear to be due to a non-specific effect, e.g. a memory deficit, as similar treatment of acute morphine animals did not reduce preference for the morphine environment. The difference in effectiveness of the different doses of fluoxetine may reflect the fact that fluoxetine dose-dependently increases forebrain 5-HT (Guan and McBride 1988; Rutter and Auerbach 1993) and therefore the lower dose may not completely reverse 5-HT changes in the chronic morphine animals. This indicates that the motivation of withdrawn animals to seek out morphine-associated stimuli may be due in part to neurochemical changes involving the 5-HT system. The fact that the morphine withdrawn rats showed significantly reduced head shaking behavior following 5-HTP administration establishes that 5-HT transmission was deficient at the time of preference testing. These findings are consistent with other reports showing evidence for reduced 5-HT transmission during morphine withdrawal (Ahtee 1980; Tao et al. 1998).
The chronic morphine rats showed a much larger preference for the morphine-paired environment than the acutely morphine-treated rats. This effect was seen whether the chronic animals were conditioned with the 10 or 20 mg/kg dose of morphine. This result indicates that the motivation driving preference for the morphine-related stimuli may be different in the chronic and acute morphine conditions. The negative reinforcement associated with alleviation of morphine withdrawal could summate with the positive reinforcing aspects of morphine in the dependent animals. Similar “negative reinforcement theories” of morphine addiction have been proposed previously (Koob and Bloom 1988; Nader and van der Kooy 1996). According to this theory, abstinence following chronic drug treatment produces a negative state (i.e., dysphoria, anhedonia, anxiety, etc.) and this negative state is mentally contrasted to the memories of the euphoric properties of morphine, increasing the likelihood that subjects will be drawn to morphine and morphine-associated stimuli.
Alternatively, other experiments have shown sensitization to the rewarding properties of morphine using the place preference paradigm (Lett 1989; Shippenberg et al. 1996, 1998; Contarino et al. 1997). In those studies as in ours, rats treated chronically with morphine showed enhanced place preference for a morphine-associated environment relative to rats conditioned acutely with morphine. Notably, unlike our experiment, rats were not made physically dependent on morphine. Therefore, the enhanced preference seen in the chronically treated animals in our study may be due to a combination of sensitization to the rewarding effects of morphine as well as the alleviation of the negative symptoms associated with morphine withdrawal.
It has been proposed that the motivational forces underlying morphine addiction may at least in part result from subjective withdrawal symptomatology (Koob and Bloom 1988). Furthermore, it has been hypothesized that the neural substrates that mediate the reinforcing actions of drugs are modified by chronic drug use and subsequently become the mechanisms that produce the anhedonic properties of drug withdrawal (Koob et al. 1989). Previous studies have found that the accumbens is a site that mediates the reinforcing properties of drugs (Dworkin et al. 1988; Pettit et al. 1984; Zito et al. 1985). In this study, the fact that fluoxetine was equally effective in decreasing the preference for the morphine environment in morphine withdrawn subjects when given in the accumbens as when given systemically provides support for the view that the accumbens may also contribute to the anhedonic properties of drug withdrawal.
For the acute morphine rats, there was no long-term change in brain 5-HT systems and no negative state produced. In these rats, the 5-HT enhancing treatments may have resembled the acute effects of morphine, which also enhances 5-HT function (Tao and Auerbach 1994, 1995) thereby cueing the animals to the morphine environment. The fact that similar enhancements in preference were produced by morphine pretreatment on the test day in the acute morphine treated rats supports this possibility. In these rats the 3.5 mg/kg dose of fluoxetine was slightly more potent in producing an enhanced preference than the 10 mg/kg dose. This may reflect a sedative effect that is known to occur at high doses of this drug (Gupta and Masand 1999).
In this study, as in a previous report (Harris and Aston-Jones 1993), morphine withdrawn rats showed profound increases in anxiety in the conditioned burying paradigm. Here, fluoxetine administration markedly decreased this morphine withdrawal-induced anxiety. Similar anxiolytic results for fluoxetine have been shown in rats using the elevated plus maze task (Griebel et al. 1999). In addition, fluoxetine is also known to have anxiolytic effects in the treatment of human anxiety disorders (Nutt et al. 1999). Because anxiety is a potent stimulator of drug cravings in human heroin addicts (Childress et al. 1994), it may also play a role in the enhanced preference for morphine cues seen in the chronic morphine rats.
Serotonin is a potent stimulator of DA release (Benloucif et al. 1993; Parsons and Justice 1993). Although speculative, it is possible that by increasing brain 5-HT levels, the fluoxetine and 5-HTP treatments used here may also stimulate the DA system, thereby enhancing the levels of both transmitters. This increased DA may attenuate some aspects of morphine withdrawal associated with depressed accumbal DA (Harris and Aston-Jones 1994; Pothos et al. 1991; Rossetti et al. 1992). Increased DA release may also contribute to the cueing by fluoxetine and 5-HTP which leads to the enhanced preference for the morphine environment seen in the acute morphine treated rats (discussed above). Although a number of reports have shown that fluoxetine in the doses used in this study has no effect on DA levels in the accumbens (Clark et al. 1996; Ichikawa and Meltzer 1995; Perry and Fuller 1992), one recent study found that similar doses of fluoxetine significantly increased DA levels in the frontal cortex (Gobert et al. 1997). The frontal cortex is one area shown to be hypofunctional during opiate abstinence and withdrawal in human opiate addicts (Danos et al. 1998; Gerra et al. 1998; Krystal et al. 1995) and it is possible that the treatments given in this study may be effective in changing preference by altering activity in this brain region.
The present data indicate that our animals exhibited emotional signs of morphine withdrawal at the time of preference testing that mimic those seen in human addicts, i.e. desire for morphine associated cues and anxiety (Jaffe 1990). Furthermore, these findings indicate that decreased 5-HT function may be associated with these subjective symptoms of morphine withdrawal. To date no treatment for morphine addiction has been satisfactory. Our results indicate that 5-HT enhancing agents may be promising treatments for morphine abuse. Understanding the role of altered 5-HT function in morphine withdrawal could be important in the design of pharmacological treatments for morphine addiction.
References
Acquas E, Carboni E, Di Chiara G . (1991): Profound depression of mesolimbic dopamine release after morphine withdrawal in dependent rats. European Journal of Pharmacology 193: 133–134
Ahtee L . (1980): Chronic morphine administration decreases 5-hydroxytryptamine and 5-hydroxyindoleacetic acid content in the brain of rats. Medical Biology 58: 38–44
Antkiewicz-Michaluk L, Romanska I, Vetulani J . (1995): Immediate and “day-after” effects of morphine on dopamine and serotonin metabolism in various structures of the rat brain. Polish Journal of Pharmacology 47: 355–358
Bardo MT, Rowlett JK, Harris MJ . (1995): Conditioned place preference using opiate and stimulant drugs: a meta-analysis. Neuroscience Biobehavioral Reviews 19: 39–51
Bedard P, Pycock CJ . (1977): ‘Wet-dog’ shake behavior in the rat: a possible quantitative model of central 5-hydroxytryptamine activity. Neuropharmacology 16: 663–670
Benloucif S, Keegan MJ, Galloway MP . (1993): Serotonin-facilitated dopamine release in vivo: pharmacological characterization. Journal of Pharmacology and Experimental Therapeutics 265: 373–377
Bloom FE, Hoffer BJ, Siggins GR, Barker JL, Nicoll RA . (1972): Effects of serotonin on central neurons: microiontophoretic administration. Federal Proceedings 31: 97–106
Bozarth MA, Wise RA . (1981): Heroin reward is dependent on a dopaminergic substrate. Life Sciences 29: 1881–1886
Boadle-Biber MC, Johannessen JN, Narasimhachari N, Phan T-H . (1987): Activation of cortical tryptophan hydroxylase by acute morphine treatment: blockade by 6-hydroxydopamine. European Journal of Pharmacology 139: 193–204
Bonci A, Williams JT . (1997): Increased probability of GABA release during withdrawal from morphine. Journal of Neuroscience 17: 796–803
Childress AR, Ehrman R, McLellan AT, MacRae J, Natale M, O'Brien CP . (1994): Can induced moods trigger drug-related responses in opiate abuse patients. Journal of Substance Abuse Treatment 11: 17–23
Clark RN, Ashby CR Jr, Dewey SL, Ramachandran PV, Strecker RE . (1996): Effect of acute and chronic fluoxetine on extracellular dopamine levels in the caudate-putamen and nucleus accumbens of rat. Synapse 23: 125–131
Contarino A, Zanotti A, Drago F, Natolino F, Lipartiti M, Giusti P . (1997): Conditioned place preference: no tolerance to the rewarding properties of morphine. Naunyn Schmiedebergs Archives of Pharmacology 355: 589–594
Danos P, Kasper S, Grunwald F, Klemm E, Krappel C, Broich K, Hoflich G, Overbeck B, Biersack HJ, Moller HJ . (1998): Pathological regional cerebral blood flow in opiate-dependent patients during withdrawal: a HMPAO-SPECT study. Neuropsychobiology 37: 194–199
Darmani NA, Shaddy J, Elder EL . (1997): Prolonged deficits in presynaptic serotonin function following withdrawal from chronic cocaine exposure as revealed by 5-HTP-induced head-twitch response in mice. Journal of Neural Transmission 104: 1229–1247
Dolberg OT, Iancu I, Sasson Y, Zohar J . (1996): The pathogenesis and treatment of obsessive-compulsive disorder. Clinical Neuropharmacology 19: 129–147
Dworkin S, Guerin GF, Goeders NE, Smith JE . (1988): Kainic acid lesions of the nucleus accumbens selectively attenuate morphine self-administration. Pharmacology, Biochemistry & Behavior 29: 175–181
Ehrman RN, Robbins SJ, Childress AR, O'Brien CP . (1992): Conditioned responses to cocaine-related stimuli in cocaine abuse patients. Psychopharmacology 107: 523–529
Ettenberg A, Pettit HO, Bloom FE, Koob GF . (1982): Heroin and cocaine intravenous self-administration in rats: mediation by separate neural systems. Psychopharmacology 78: 204–209
Fuller RW, Wong DT, Robertson DW . (1991): Fluoxetine, a selective inhibitor of serotonin uptake. Medical Research Reviews 11: 17–34
Garcia-Sevilla JA, Magnusson T, Carlsson A . (1980): Effects of enkephalins and two enzyme resistant analogues on monoamine synthesis and metabolism in rat brain. Naunyn Schmiedebergs Archives of Pharmacology 310: 211–218
Gerra G, Calbiani B, Zaimovic A, Sartori R, Ugolotti G, Ippolito L, Delsignore R, Rustichelli P, Fontanesi B . (1998): Regional cerebral blood flow and comorbid diagnosis in abstinent opioid addicts. Psychiatry Research 83: 117–126
Gerra G, Fertonani G, Zaimovic A, Rota-graziosi I, Avanzini P, Caccavari R, Delsignore R, Lucchini A . (1995): Hostility in heroin abusers subtypes: fluoxetine and naltrexone treatment. Progress in Neuropsychopharmacology and Biological Psychiatry 19: 1225–1237
Gerrits MA, Van Ree JM . (1996): Effect of nucleus accumbens dopamine depletion on motivational aspects involved in initiation of cocaine and heroin self-administration in rats. Brain Research 713: 114–124
Gobert A, Rivet JM, Cistarelli JM, Milllan MJ . (1997): Buspirone enhances duloxetine- and fluoxetine-induced increases in dialysate levels of dopamine and noradrenaline, but not serotonin, in the frontal cortex of freely moving rats. Journal of Neurochemistry 68: 1326–1329
Gold LH, Stinus L, Inturrisi CE, Koob GF . (1994): Prolonged tolerance, dependence and abstinence following subcutaneous morphine pellet implantation in the rat. European Journal of Pharmacology 253: 45–51
Griebel G, Cohen C, Perrault G, Sanger DJ . (1999): Behavioral effects of acute and chronic fluoxetine in Wistar-Kyoto rats. Physiology and Behavior 67: 315–320
Guan X-M, McBride WJ . (1988): Fluoxetine increases the extracellular levels of serotonin in the nucleus accumbens. Brain Research Bulletin 21: 43–46
Gudelsky GA, Nash JF . (1996): Carrier-mediated release of serotonin by 3,4-methylenedioxymethamphetamine: implications for serotonin-dopamine interactions. Journal of Neurochemistry 66: 243–249
Gupta S, Masand PS . (1999): Selective serotonin-reuptake inhibitors: an update. Harvard Review of Psychiatry 7: 69–84
Harris GC, Aston-Jones G . (1993): β-adrenergic antagonists attenuate withdrawal anxiety in cocaine- and morphine-dependent rats. Psychopharmacology 113: 131–136
Harris GC, Aston-Jones G . (1994): Involvement of D2 dopamine receptors in the nucleus accumbens in the opiate withdrawal syndrome. Nature 371: 155–157
Ichikawa J, Meltzer HY . (1995): Effect of antidepressants on striatal and accumbens extracellular dopamine levels. European Journal of Pharmacology 281: 255–261
Jaffe JH . (1990):. In: Gilman AG, Rall TW, Nies AS, Taylor P (eds) Goodman and Gilman's The Pharmacological Basis of Therapeutics. Pergamon Press, New York, pp 522–573
Koob GF, Bloom FE . (1988): Cellular and molecular mechanisms of drug dependence. Science 242: 715–723
Koob GF, Rocio M, Carrera MRA, Gold LH, Heyser CJ, Maldonado-Irizarry C, Markou A, Parsons LH, Roberts AJ, Schulteis G, Stinus L, Walker JR, Weissenborn R, Weiss F . (1998): Substance dependence as a compulsive behavior. Journal of Psychopharmacology 12: 39–48
Koob GF, Stinus L, Le Moal ML, Bloom FE . (1989): Opponent process theory of motivation: neurobiological evidence from studies of opiate dependence. Neuroscience and Biobehavioral Reviews 13: 135–140
Krystal JH, Woods SW, Kosten TR, Rosen MI, Seibyl JP, Van Dyck CC, Price LH, Zubal IG, Hoffer PB, Charney DS . (1995): Opiate dependence and withdrawal: preliminary assessment using single photon emission computerized tomography (SPECT). Journal of Drug and Alcohol Abuse 21: 47–63
Lett BT . (1989): Repeated exposures intensify rather than diminish the rewarding effects of amphetamine, morphine, and cocaine. Psychopharmacology 98: 357–362
Lucki I, Nobler MS, Frazer A . (1984): Differential actions of serotonin antagonists on two behavioral models of serotonin receptor activation in the rat. Journal of Pharmacology and Experimental Therapeutics 228: 133–139
Mackey WB, van der Kooy D . (1985): Neuroleptics block the positive reinforcing effects of amphetamine but not of morphine as measured by place conditioning. Pharmacology Biochemistry and Behavior 22: 101–105
Maes M, Meltzer HY . (1995): The serotonin hypothesis of major depression. In Bloom FE, Kupfer DJ (eds), Psychopharmacology: The Fourth Generation of Progress. Raven Press, Raven Press, pp 933–944
Maremmani I, Castrogiovanni P, Daini L, Zolesi O . (1992): Use of fluoxetine in heroin addiction. British Journal of Psychiatry 160: 570–571
Markou A, Kosten TR, Koob GF . (1998): Neurobiological similarities in depression and drug dependence: a self-medication hypothesis. Neuropsychopharmacology 18: 135–174
Nader K, van der Kooy D . (1996): Clonidine antagonizes the aversive effects of opiate withdrawal and the rewarding effects of morphine only in opiate withdrawn rats. Behavioral Neuroscience 110: 389–400
Nutt DJ, Forshall S, Bell C, Rich AR, Sandford J, Nash J, Argyropoulos S . (1999): Mechanisms of action of selective serotonin reuptake inhibitors in the treatment of psychiatric disorders. European Neuropsychopharmacology 9(Suppl. 3): S81–S86
O'Brien CP, Childress AR, Ehrman R, Robbins SJ . (1998): Conditioning factors in drug abuse: can they explain compulsion? Journal of Psychopharmacology 12: 15–22
Parsons LH, Justice JB Jr . (1993): Perfusate serotonin increases extracellular dopamine in the nucleus accumbens of the rat as measured by in vivo microdialysis. Brain Research 606: 195–199
Perry KW, Fuller RW . (1992): Effect of fluoxetine on serotonin and dopamine concentration in microdialysis fluid from rat striatum. Life Science 50: 1683–1690
Perry KW, Fuller RW . (1993): Extracellular 5-hydroxytryptamine concentration in rat hypothalamus after administration of fluoxetine plus L-5-hydroxytryptophan. Journal of Pharmacy and Pharmacology 45: 759–761
Pettit HO, Ettenberg A, Bloom FE, Koob GF . (1984): Destruction of dopamine in the nucleus accumbens selectively attenuates cocaine but not heroin self-administration in rats. Psychopharmacology 84: 167–173
Pothos E, Rada P, Mark GP, Hoebel BG . (1991): Dopamine microdialysis in the nucleus accumbens during acute and chronic morphine, nalxone-precipitated withdrawal and clonidine treatment. Brain Research 566: 348–350
Rockman GE, Amit Z, Bourque C, Brown ZW, Ogren SO . (1980): Reduction of voluntary morphine consumption following treatment with zimelidine. Archives of International Pharmacodynamics and Therapy 244: 123–129
Rossetti ZL, Hmaidan Y, Gessa GL . (1992): Marked inhibition of mesolimbic dopamine release: a common feature of ethanol, morphine, cocaine and amphetamine abstinence in rats. European Journal of Pharmacology 221: 227–234
Rutter JJ, Auerbach SB . (1993): Acute uptake inhibition increases extracellular serotonin in the rat forebrain. The Journal of Pharmacology and Experimental Therapeutics 265: 1319–1324
Shippenberg TS, Heidbreder C, LeFevour A . (1996): Sensitization to the conditioned rewarding effects of morphine: pharmacology and temporal characteristics. European Journal of Pharmacology 299: 33–39
Shippenberg TS, LeFevour A, Thompson AC . (1998): Sensitization to the conditioned rewarding effects of morphine and cocaine: differential effects of the kappa-opioid receptor agonist U69593. European Journal of Pharmacology 345: 27–34
Smith JE, Geurin GF, Co C, Barr TS, Lane JD . (1985): Effects of 6-OHDA lesions of the medial nucleus accumbens on rat intravenous morphine self-administration. Pharmacology Biochemistry and Behavior 23: 843–849
Smith JE, Shultz K, Co C, Goeders NE, Dworkin SI . (1987): Effects of 5,7-dihydroxytryptamine lesions of the nucleus accumbens on rat intravenous morphine self-administration. Pharmacology Biochemistry and Behavior 26: 607–612
Spampinato U, Esposito E, Romandini S, Samanin R . (1985): Changes of serotonin and dopamine metabolism in various forebrain areas of rats injected with morphine either systemically or in the raphe nuclei dorsalis and medianus. Brain Research 328: 89–95
Spyraki C, Fibiger HC, Phillips AG . (1983): Attenuation of heroin reward in rats by disruption of the mesolimbic dopamine system. Psychopharmacology 79: 278–283
Spyraki C, Nomikos GG, Galanopoulou P, Daifotis Z . (1988): Drug-induced place preference in rats with 5,7-dihydroxytryptamine lesions of the nucleus accumbens. Behavioral Brain Research 29: 127–134
Tao R, Auerbach SB . (1994): Increased extracellular serotonin in rat brain after systemic or intraraphe administration of morphine. Journal of Neurochemistry 63: 517–524
Tao R, Auerbach SB . (1995): Involvement of the dorsal raphe but not the median raphe nucleus in morphine-induced increases in serotonin release in the rat forebrain. Neuroscience 68: 553–561
Tao R, Ma Z, Auerbach SB . (1998): Alteration in regulation of serotonin release in rat dorsal raphe nucleus after prolonged exposure to morphine. Journal of Pharmacology and Experimental Therapeutics 286: 481–488
Treit D . (1985): Animal models for the study of anti-anxiety agents: a review. Neuroscience Biobehavioral Reviews 9: 203–222
Treit D, Pinel JPJ, Fibiger HC . (1981): Conditioned defensive burying: a new paradigm for the study of anxiolytic agents. Pharmacology Biochemistry and Behavior 15: 619–626
Tzschentke TM . (1998): Measuring reward with the conditioned place preference paradigm: a comprehensive review of drug effects, recent progress and new issues. Progress in Neurobiology 56: 613–672
Wang Y, Joharchi N, Fletcher PJ, Sellers EM, Higgins GA . (1995): Further studies to examine the nature of dexfenfluramine-induced suppression of heroin self-administration. Psychopharmacology 120: 134–141
Wise RA, Leone P, Rivest R, Leeb K . (1995): Elevations of nucleus accumbens dopamine and DOPAC levels during intravenous heroin self-administration. Synapse 21: 140–148
Yoburn BC, Chen J, Huang T, Inturrisi CE . (1985): Pharmacokinetics and pharmacodynamics of subcutaneous morphine pellets in the rat. Journal of Pharmacology and Experimental Therapeutics 235: 282–286
Zito KA, Vickers G, Roberts DCS . (1985): Disruption of cocaine and heroin self-administration following kainic acid lesions of the nucleus accumbens. Pharmacology Biochemistry and Behavior 23: 1029–1036
Acknowledgements
This work was supported by PHS grant DA-06214.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Harris, G., Aston-Jones, G. Augmented Accumbal Serotonin Levels Decrease the Preference for a Morphine Associated Environment During Withdrawal. Neuropsychopharmacol 24, 75–85 (2001). https://doi.org/10.1016/S0893-133X(00)00184-6
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1016/S0893-133X(00)00184-6
Keywords
This article is cited by
-
Response dynamics of midbrain dopamine neurons and serotonin neurons to heroin, nicotine, cocaine, and MDMA
Cell Discovery (2018)
-
Cocaine Seeking During Initial Abstinence Is Driven by Noradrenergic and Serotonergic Signaling in Hippocampus in a Sex-Dependent Manner
Neuropsychopharmacology (2017)
-
κ-opioid receptor as a key mediator in the regulation of appetitive 50-kHz ultrasonic vocalizations
Psychopharmacology (2015)
-
Dissociation of heroin-induced emotional dysfunction from psychomotor activation and physical dependence among inbred mouse strains
Psychopharmacology (2015)
-
Distinct Mu, Delta, and Kappa Opioid Receptor Mechanisms Underlie Low Sociability and Depressive-Like Behaviors During Heroin Abstinence
Neuropsychopharmacology (2014)