Abstract
Recent biochemical data suggest that arachidonylethanolamide (AEA; anandamide) may be an endogenous ligand for brain cannabinoid receptors. The functional neuronal consequences of AEA binding to cannabinoid receptors are only poorly understood. Using regional cerebral blood flow (rCBF) as an indirect marker of neuronal activity, acute AEA administration dose-dependently depressed rCBF in unanesthetized rats. Although 3.0 mg/kg was ineffective in altering rCBF, 10 mg/kg led to a decrease in rCBF in seven brain areas including the amygdala, cingulate, frontal, prepyriform, sensorimotor, and claustrocortex. An additional 16 areas responded in a similar manner to AEA, but only after 30 mg/kg, including the CA1 and CA3 regions of the hippocampus, the rostral core portion of the nucleus accumbens, and rostral caudate nucleus. Most of these rCBF effects dissipated between 15 and 20 min after drug administration, with only 4 regions, the basomedial and lateral amygdala, CA3 hippocampus and claustrocortex still depressed 60 min after an acute drug injection. No significant changes in heart rate, blood pressure, or blood gases were seen at the time of rCBF measurement, suggesting that the observed drug effects were neuronally mediated. Taken together with existing behavioral data, these data support the hypothesis that an endogenous cannabinoid neural system exists in mammalian brain and may help to explain the unique behavioral profile seen after cannabinoid administration.
Similar content being viewed by others
Main
The behavioral profile seen in humans following administration of marijuana has long been a source of intense interest, because the unique syndrome produced does not resemble that seen after any other psychoactive substance. Subjectively, the cannabinoid syndrome in humans includes sensory enhancement, errors in the judgment of time and space, dissociation of ideas, delusions, impulsivity, and hallucinations (Pertwee 1988). For example, individuals often report that while under the influence of cannabis, auditory, gustatory, and visual stimuli often seem more intense and pleasurable; whereas, time often seems to proceed more slowly. Memory loss, especially short-term or working memory, and attentional deficits are also hallmarks of cannabinoid intoxication (Chait and Pierri 1992; Schwartz 1993). The central nervous system mechanisms and sites of action of these cognitive effects are still poorly understood.
Regional changes in cerebral blood flow and metabolism are thought to reflect changes in regional neuronal activity (Sokoloff 1981). Because specific cognitive or drug-induced challenges are known to induce regionally specific changes in neuronal activity regional cerebral blood flow (rCBF), and glucose utilization (Sokoloff, 1981), various functional metabolic imaging procedures have been employed in an attempt to understand better marijuana's neuroanatomical sites of action. Mathew and colleagues (Mathew et al. 1989, 1992) measured changes in rCBF following acute drug administration. An increase in CBF was seen in experienced versus inexperienced subjects with greater frontal and right hemisphere blood flow seen 30 min after marijuana smoking. These blood flow effects were not related to changes in general circulation, respiration, or plasma tetrahydrocannabinol (THC) concentrations (Mathew and Wilson 1993). In contrast, using intravenous Δ9 THC, the principal psychoactive agent in marijuana, Volkow et al. (1991) reported an increase in glucose utilization (rCMRglu) only in the cerebellum when data were corrected for changes in global metabolism. More recently, in a population of THC abusers, Volkow et al. (1996) demonstrated THC-induced increases in rCMRglu in the orbitofrontal and prefrontal cortex and basal ganglia that correlated with the subjective sense of intoxication. Baseline cerebellar rCMRglu rates were also lower in abusers than control subjects. In contrast to the above studies on human subjects, Margulies and Hammer (1991) reported biphasic, dose-dependent effects of Δ9 THC in most limbic (but not diencephalic/brainstem) regions, with low doses causing an increase and high doses a decrease in rCMRglu. We (Bloom et al. 1997) have also demonstrated heterogenous decreases in rCBF after acute Δ9 THC administration in the rat at doses comparable to the high doses of Margulies and Hammer (1991).
Despite these gains in our understanding of the neuroanatomical localization of Δ9 THC's effect upon brain activity, the recent discovery of an endogenous cannabinoid receptor in mammalian brain (Devane et al. 1988; Howlett et al. 1990; Matsuda et al. 1993) and the existence of at least one endogenous compound that binds to that receptor (Devane et al. 1992), has prompted re-examination of the system. Arachidonylethanolamide (anandamide; AEA) binds to rat brain membranes (Hillard et al. 1995), inhibits the binding of synthetic cannabinoids to the receptor (Devane et al. 1992), inhibits calcium currents in neuroblastoma cells (Mackie and Hille 1992), and inhibits forskolin stimulated adenylate cyclase activity in COS cells (Vogel et al. 1993) and rat brain membranes (Childers et al. 1994). Several subsequent reports have suggested that AEA possesses biological activity similar to that of Δ9 THC. For example, AEA depresses locomotor behavior, causes a mild hypothermia, produces antinociception, and has profound, but short-lived, cardiovascular effects (Crawley et al. 1993; Fride and Mechoulam 1993; Smith et al. 1994; Stein et al. 1996; Varga et al. 1995). However, there are little data to suggest whether AEA possesses psychoactive properties. Because the rat has been extensively used to determine the behavioral properties of both Δ9 THC and AEA, and because these drug-induced behavioral actions are likely induced by altered neuronal activity within specific brain regions, we sought to determine the profile of brain activity after acute AEA administration in the rat. It was hypothesized that the pattern of drug-induced rCBF alterations would include limbic and sensorimotor regional alterations, reflecting the unique behavioral profile induced by Δ9 THC and AEA.
Because intravenous AEA is known to have a rapid onset and a relatively brief duration of action (Stein et al. 1996), and because under normal physiological conditions, increases in neural activity are tightly coupled to increased microcapillary perfusion and local delivery of energy substrates (Busija and Heistad 1984), we employed an autoradiographic procedure to measure changes in rCBF (Sakurada et al. 1978) to determine the effects of an acute, intravenous AEA injection on regional brain activity in the rat.
MATERIALS AND METHODS
Forty-four male, Sprague–Dawley derived rats (Sasco, Madison, WI, USA), weighing 250 to 325 g were group housed in plastic tubs in a temperature controlled room with lights off between 0830 to 2030 hrs. All experiments and behavioral manipulations took place during the rat's dark (active) phase under ambient light conditions. Food and water were available ad libitum. Prior to blood flow determination, each rat experienced a restraint procedure of increasing duration for 5 days, progressing from 1 to 5 h/day. The restraint consisted of gently immobilizing both fore and hind limbs by wrapping the rat in a terry cloth towel. Rats rapidly acclimated to this restraint, ceased to struggle and would accept food and water if offered.
On the day of sacrifice, rats were anesthetized with sodium pentobarbital (50 mg/kg), and femoral arterial and venous catheters (PE10) were implanted into the left leg and a second arterial catheter in the right leg. Immediately following surgery, animals were placed into the restraint and allowed to recover from anesthesia for a minimum of 5 h. An intravenous injection of 500 IU/kg heparin was delivered in 0.5 ml saline 1 h prior to drug treatment.
Groups of rats received one of four drug treatments in 0.5 ml saline: either (1) saline or AEA at a dose of; (2) 3.0 mg/kg; (3) 10.0 mg/kg; or (4) 30.0 mg/kg. All injections were made by hand into the femoral vein over a period of approximately 30 s and followed by 0.3 ml saline to flush the catheter. For the dose-response study, rCBF measurement commenced 15 min after drug or vehicle treatment; in the time course experiment, groups of rats received 30.0 mg/kg AEA and were sacrificed 15, 20, and 60 min post drug administration.
RCBF was measured by the method of Sakurada et al. (1978). Briefly, this autoradiographic method involved the infusion of a 0.5 ml saline solution containing 100 μCi/kg [14C]-IAP (45.5 mCi/mmol, Amersham) at a constant rate over 30 s into the femoral vein. Arterial blood samples (approximately 20–30 μl) were collected every 5 s onto preweighed filter paper during the entire 30 s infusion period. Blood flowed freely from the 6 to 7 mm length of PE 10 tubing, which had a dead space of approximately 3 to 4 μl. At the end of the isotope infusion period, rats were sacrificed by rapid decapitation and the entire skull, less jaw and fur, was frozen in isopentane (−50°C) and stored at −80°C until sectioned.
Filter papers were immediately sealed in 7 ml counting vials and reweighed. Radioactivity was determined by liquid scintillation spectroscopy (Beckman LS6000TA counter) 24 hr after the addition of 5 ml Budget Solve (RPI, Mt. Prospect, IL, USA). Vials were vigorously vortexed several times to facilitate the elution of [14C] IAP from the filter papers.
Brains were removed from skulls in a cryostat (Reichert-Jung 1800) and subsequently sectioned into 20 μm slices in the coronal plane at −20°C, thaw mounted onto glass slides, dried on a slide warmer, and apposed to x-ray film (Fuji UM-MA) in standard cassettes (Wolf) with calibrated [14C] methyl methacrylate standards (Amersham) for up to 2 months. Following film development, slides were stained with thionin for subsequent anatomic localization of regions of interest (ROI). Brain regions were analyzed on an MCID Image Analyzer (Imaging Research, St. Catherines, ON, Canada) with ROIs defined using the comparable stained section as defined by the atlas of Paxinos and Watson (1986). Five evenly spaced, bilateral densitometric measurements were taken for each region analyzed and averaged together for each rat.
Regional cerebral blood flow (ml/100 g/min) was calculated on-line from the operational equation of Sakurada et al. (1978) using the arterial radioactivity concentration curves and film optical density calibration data to reflect final tissue concentration of radioactivity. No correlation for catheter dead space was made, because Jay et al. (1988) demonstrated that catheter flow rates at least 40 times the dead space volume accurately represented brain blood flow (our ratios generally ranged from 60–90 times dead space).
Concurrent with rCBF determination, heart rate and mean arterial, diastolic, and systolic blood pressure were determined on-line by means of a pressure transducer (Cobe Instruments, Arvada, CO, USA) and a CODAS digital polygraph (Dataq Instruments, Columbus, OH, USA) connected to the right femoral arterial catheter. Timed arterial blood samples were collected prior to drug injection and again immediately preceding rCBF determination and sacrifice. Samples were subsequently analyzed for pH, PaCO2, PaO2, and HCO3− using a blood gas analyzer (Model 995, AVL Scientific, Roswell, GA, USA). Drug-induced behavioral assessments were conducted during the post-drug period by two independent observers who were blinded to the treatment each rat received. Catatonia was assessed visually following placement of the rat's fore-and hind-limbs in various positions and noting their return to previous location. Brief loud tone pulses and airpuffs to the face were randomly presented to each rat, and the animal's response (vocalization, locomotion, orienting, reflexia) was rated.
AEA was synthesized from arachidonyl chloride following the method of Devane et al. (1992), and stored under N2 at −20°C until used. Briefly, arachidonic acid (AA) was added to dry dichloromethane with 1.2 equivalents of oxalyl chloride in the presence of 1 equivalent of dimethylformamide at 0°C to form the acid chloride of AA. After 15 min, the acid chloride was added to a 10-fold excess of ethanolamine and incubated for 60 min at 0°C. The reaction mixture was washed with water followed by removal of the solvent under N2. AEA was separated from arachidonic acid using thin layer chromatography on silica gel HL plates developed with hexane/ethyl acetate/methanol (60/40/5) and stored under N2 at −20°C until used. Purity and identity of AEA was established by NMR and mass spectrometry. The drug was prepared fresh each day in an emulphor–ethanol vehicle (1:1) and diluted with saline to maintain a constant injection volume of 0.5 ml. The physiological characterization of AEA has been previously described (Stein et al. 1996).
A multvariate analysis of variance (MANOVA) of treatment × variable × time (within subjects pre-post drug) was performed on the physiologic/blood gas data. To assess the acute effects of AEA, a two-way analysis of variance (ANOVA) of treatment × drug was performed on the heart rate and blood pressure data. Differences between rCBF treatment groups were evaluated by individual one-way ANOVAs for each brain area measured. A modified Bonferonni's correction was applied because of the large number of regions analyzed. As such, an α level of 0.001 was chosen for the rCBF ANOVAs. Post-hoc comparisons were performed, where appropriate, using the Neuman-Keuls’ Multiple Range Test. A significance level of 0.05 was used for all other physiological and rCBF measures.
RESULTS
The effects of acute AEA on physiologic parameters are presented in Table 1. Analyses of variance performed on both the dose-response and time-course groups indicated no significant alterations in any of the blood gas parameters monitored, nor did the drug have an effect on heart rate or mean, systolic, or diastolic blood pressure when measured immediately prior to rCBF determination. This is not to say that AEA had no effect on these physiological systems. We and others (Crawley et al. 1993; Stein et al. 1996; Varga et al. 1995) have reported dramatic, short-term alterations in heart rate and blood pressure following acute AEA administration. However, at the time of sacrifice (15, 20, or 60 min), these systemic effects had long since returned to baseline values.
Although mildly restrained in a towel, intravenous AEA administration exerted profound, brief duration, dose-related behavioral effects in the unanesthetized rat. Administration of either 10 or 30 mg/kg AEA produced an immediate waxy catatonia; rats receiving the high dose became ataxic and often lost their righting reflex. This behavioral state lasted for about 14 min following 30 mg/kg and 5 min following the 10.0 mg/kg dose group. In contrast, the 3 mg/kg dose produced virtually no behavioral depression, and it was difficult to identify this group from vehicle controls on the basis of visual inspection.
In spite of their catatonia, rats displayed a hypersensitivity to many environmental stimuli including auditory and somatosensory stimulation. Brief, loud sounds (a tone pulse) induced reflexive vocalization and escape-like responses. Rats would immediately orient to the stimulus, only to return to the cataleptic-like state in the absence of any external stimuli. This hyperreflexia was similar to the behavior seen in rodents after THC administration (Dewey 1986). However, in contrast to the immediate onset of catatonia, the hyperreflexia had a more delayed onset; for the high dose, a delay of between 5 to 10 min ensued with a duration of effect that averaged more than 20 min (range 14–43 min). At 10 mg/kg, onset varied from 2 to 5 min and lasted for about 15 min (range 8–35 min); whereas, the hyperreflexia following 3.0 mg/kg AEA was quite mild and only occasionally seen.
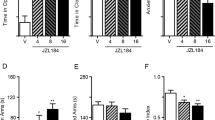
The effects of acute AEA administration on rCBF are given in Table 2. Analyses of variance for the dose-response and time-course treatment groups revealed a heterogeneous, dose- and time-dependent depression in rCBF. No increases in rCBF were ever observed at any time point or after any dose of AEA in any brain region measured. When determined 15 min after drug administration, the lowest dose of AEA had no effect on brain activity in any of the measured regions as assessed by rCBF. However, following 10.0 mg/kg AEA, 7 of 59 areas demonstrated reduced blood flow. These regions included the basomedial and lateral anygdaloid nuclei, and five cortical areas: cingulate, frontal, agranular preinsular (claustrocortex; AIP), prepyriform, and primary auditory (TE1) cortex. Blood flow in most of these regions was significantly reduced further after 30 mg/kg AEA (except in caudal AIP and TE1); whereas, 16 additional regions were effected only at the highest dose. Included in this latter group were the hippocampus (CA1 and CA3), nucleus accumbens, caudate nucleus, diagonal band of Broca, and all eight of the amygdaloid nuclei measured. Finally, large regions of the neuraxis were apparently unaffected in the current paradigm, including all measured regions of the thalamus, hypothalamus and subthalamus, the olfactory tubercle, ventral tegmental area, substantia nigra, and the shell portion of the nucleus accumbens. Figure 1 illustrates the dose-dependent properties of AEA on selected brain regions.
Dose-response effects of anandamide-induced alterations in rCBF in representative brain regions. Data are mean ± SEM rCBF (ml/100g/min). In each region altered by AEA, the 10.0 mg/kg dose significantly depressed rCBF from saline baseline (and the 3.0 mg/kg group); whereas, 30.0 mg/kg further significantly reduced rCBF compared to the middle dose. Data from the NAS shell region is illustrated to demonstrate a response that was not significantly altered by AEA. (*) denotes different from saline. (#) indicates different from all other treatment groups
Separate groups of rats received 30 mg/kg AEA and were sacrificed at 15, 20, and 60 min post drug administration. Analyses of variance indicated that of those regions depressed by AEA (see Table 2), only four (basomedial and lateral amygdala, CA3 region of the hippocampus, and the AIP) remained depressed 60 min after AEA administration. In all other regions affected, drug action dissipated sometime between the 20 and 60 min time points. There were no differences between the 15 and 20 min groups.
DISCUSSION
Acute AEA administration produced a dose-dependent reduction in rCBF in the rat brain. Together with the absence of significant drug-induced changes in cardiovascular and blood gas levels at the time of rCBF measurement, and in view of the known coupling between neuronal activity, local cerebral metabolic activity and blood flow (Sokoloff et al. 1977), these data suggest that AEA heterogeneously depressed local brain activity. Although the threshold to the AEA effect was seen at 10 mg/kg when measured 15 min after drug administration, the majority of affected brain structures were depressed only following the 30 mg/kg dose. AEA's duration of effect lasted somewhere between 20 and 60 min in most of these regions; only four were still depressed at 60 min. Finally, consistent with the unique behavioral profile seen after THC and AEA administration, the distribution of brain structures altered was unlike that seen after any other abused substance (Porrino et al. 1988; Stein and Fuller 1992; Trusk and Stein 1987) and provides insight into the sites and mechanisms underlying the behavioral profile of AEA. The significance of depressed rCBF (or neuronal activity for that matter), is still unknown in many cases, because inhibition of inhibitory processes may result in an opposite polarity of effect. Thus, the most parsimonious conclusion from these data are that AEA has an apparent physiological role in modulating the function of these structures. The discussion below outlines some of the key regional findings and how AEA may play a role in their function.
In animal models, Δ9 THC administration alters locomotor behavior, induces hypothermia, antinociception, static ataxia, and is anticonvulsant (Dewey 1986). Hyperresponsivity to auditory and tactile stimulation is also characteristic in both rats and mice (Dewey 1986; Ferri et al. 1981). Although mildly restrained, a similar behavioral profile was noted in the present study and has been reported by others after acute AEA administration in rodents (Crawley et al. 1993, Fride and Mechoulam 1993; Smith et al. 1994, Stein et al. 1996, Varga et al. 1995). The use of barbiturates to anesthetize our animals for catheter implantation prior to AEA delivery and rCBF determination is not thought to have interfered with the experimental outcome. At least 5 h elapsed from surgery to AEA administration (enough time for anesthetic metabolism), and no noticeable differences could be discerned between the behavioral responses to AEA in these animals compared to our previous report using unanesthetized rats (Stein et al. 1996). Furthermore, we (Stein and Fuller 1992) and others (Sakurada et al. 1978) have used this procedure in the past, and rCBF values from saline injected control animals in the present study are in general agreement with previously published baseline data.
Catalepsy is a hallmark of both Δ9 THC and AEA administration in rodents and can be generated after either peripheral (Ferri et al. 1981; Pertwee 1972) or direct central administration into the caudate nucleus (Gough and Olley 1978), but not the globus pallidus (Pertwee and Wickens 1991). Although both the pallidum and caudate nuclei possess dense cannabinoid receptors (Herkenham et al. 1991b), functional measures have yielded disparate results. We previously reported that Δ9THC depressed rCBF in the pallidum (Bloom et al. 1997). In the present study, IV AEA depressed caudate nucleus rCBF, but only after the highest dose administered. Methodological differences such as dose equivalence and measurement time may account for this distinction. The duration of action of AEA is significantly shorter than THC, and its potency has been estimated to be 4 to 20 times less than Δ9 THC (Smith et al. 1994). Also, cannabinoid receptors, whether found on local interneurons or projection cells, may lead to different cellular responses when activated by AEA versus Δ9THC. Such functional differences may help to explain some of the distinct motor related deficits seen after administration of each of the two drugs.
Both AEA and cannabis possess moderate analgesic/antinociceptive properties that act through both spinal and supraspinal mechanisms (Lichtman and Martin 1991; Martin et al. 1993; Smith and Martin 1992; Stein et al. 1996; Welch et al. 1994). Among its mechanisms of action, Δ9 THC is thought to modulate pain threshold by altering the perception, and/or the affective appreciation of the sensory stimulus (Noyes et al. 1975). Several of the structures depressed in this study have been implicated in sensory reception or perceptual processing and may be candidate structures for cannabinoid-induced antinociception including the primary somatosensory cortex, cingulate, calustrocortex, and amygdala. Other brain regions where cannabinoid receptors have been implicated in antiocicpetion, such as the brainstem and spinal cord, were not measured in this study.
THC is known to impair cognitive performance in humans, especially on tasks requiring either time perception or memory and learning tasks engaging working memory and free recall (Chait and Pierri 1992). In rodents, impairment on such tasks as delayed match to sample and radial arm maze have been reported following Δ9 THC (Heyser et al. 1993; Lichtman et al. 1995), although AEA failed to impair memory on either of these tasks (Crawley et al. 1993). The hippocampus has long been thought to play a prominent role in memory processes, especially declarative memory formation (Squire 1987). High concentrations of cannabinoid receptors are seen in the rat hippocampus (Herkenham et al. 1991a; Jansen et al. 1992; Thomas et al. 1992), and AEA synthesis is greatest in this area (Devane and Axelrod 1994). Heyser et al. (1993) reported decreases in hippocampal cell firing along with working memory impairment following THC. The rodent hippocampus also has been implicated in spatial processing, because cells in this area fire when animals are in a particular place in its environment (O'Keefe 1979). THC is well known for its spatial perceptual alterations. The current finding that AEA decreases rCBF in hippocampal regions CA1, CA3, and entorhinal cortex and that drug induced alterations in CA3 last at least 60 min after drug administration is consistent with these behavioral and biochemical observations. Moreover, one of the principal targets of the rat hippocampus and entorhinal cortex is the cingulate cortex (Swanson 1981), a structure also demonstrating depressed rCBF after AEA. However, we must be cautious in attributing causation in this circuit, because these regions are prominently reciprocally connected (Finch 1993).
Marijuana is known for its ability to alter temporal and spatial motor perception and performance. One manifestation of this impairment is the high incidence of vehicular motor accidents reported in THC-intoxicated individuals (Moskowitz 1985). The cingulate cortex receives important afferents from numerous cortical regions including motor, auditory, and visual regions (Finch 1993). We observed reduced rCBF at both 10 and 30 mg/kg in sensorimotor cortex in the present study. These pathways may provide a neuroanatomical substrate upon which THC and AEA exert an influence on the association between sensory inputs and spatial-motor processing.
The structure most affected by AEA in this study was the amygdala, part of the limbic system and located in the temporal lobe. Among other functions, it has been hypothesized that the amygdala processes or responds to the motivational aspects of sensory stimuli (Gloor 1975). Together with the nucleus accumbens (NAS), the basal amygdala has been linked to a network subserving, on the one side, stimulus-reward and/or punishment processes (Cador et al. 1989) and on the other, motor behavior subserving biological drives (Mogenson et al. 1980). Seven specific subnuclei within the amygdala demonstrated significant reductions in rCBF with two, the basomedial and lateral nuclei, among those brain regions revealing both the highest sensitivity to AEA and the longest duration of drug effect. The amygdaloid complex has direct and indirect connections with both the hippocampus and cingulate (Finch 1993) as well as prominent reciprocal connections with the thalamus, basal ganglia, hypothalamus, temporal cortex, and septal area (Martin et al. 1993). Specific basolateral amygdaloid complex afferents project to the prefrontal and claustrocortex (Krettek and Price 1977) and both the ventral and dorsal striatum (Kelley et al. 1982). It is noteworthy that all of these regions (i.e., frontal and AIP cortex, caudate and NAS) were significantly altered following AEA, and together with the amygdala, seem to comprise both a neuroanatomical and functional circuit that may play a role in the reinforcing properties of the cannabinoids.
Although the pharmacokinetics of THC probably are responsible for the difficulty in successfully employing the animal self-administration model of drug reinforcement, the widespread use of marijuana in society speaks to the drug's reinforcing properties (Compton et al. 1996). Data reported over the years by Gardner and colleagues support the hypothesis that mesocorticolimbic dopamine pathways are activated by THC much like other drugs of abuse (Wise and Rompre 1989). Δ9 THC facilitates reinforcing electrical brain stimulation (Gardner and Lowinson 1991; Gardner et al. 1988) and enhances dopamine transmission (Chen et al. 1993; Chen et al. 1990). A relatively high concentration of cannabinoid receptors is seen in the NAS (Herkenham et al. 1991b; Jansen et al. 1992; Thomas et al. 1992), while dopamine efflux in the NAS is increased by THC (Chen et al. 1990). In the present study, AEA administration significantly depressed rCBF in the rostral core portion of the NAS, cingulate, and frontal cortex as well as much of the amygdaloid complex. In view of the anatomic and functional significance of these regions in processing the affective components of stimuli, these regions may be target candidates for responding to the putative motivational properties of AEA. It is notable that such brain regions as the ventral tegmentum, olfactory tubercle, NAS shell, and lateral hypothalamus that have been previously implicated in processing the reinforcing properties of other abused drugs, including cocaine and opiates, and natural reinforcers such as food, water, and sexual behaviors (Wise and Rompre 1989), were not significantly effected by AEA in the current experiment. Thus, although AEA's influence on reward processes has not yet been extensively studied, it would be of great interest to examine the interaction of AEA with dopaminergic mechanisms in these areas.
In sum, results from this study provide direct evidence that acute intravenous AEA administration can heterogeneously depress specific rat brain loci. Many of the brain regions identified in this study have either been previously shown to respond to Δ9 THC administration, contain moderate to high amounts of cannabinoid receptors and/or have been implicated in many of the behavioral properties of Δ9 THC. Taken together with previous reports of the existence of brain cannabinoid receptors and the enzymes to synthesize and catabolize AEA (Deutsch and Chin 1993), these data are consistent with the hypothesis that AEA may serve as a neurotransmitter or neuromodulator of brain functions. Additional drug doses and analytic methods, together with the development of specific receptor antagonists should provide further insights under what normal and/or pathological conditions AEA is synthesized and released and from which brain structures.
References
Bloom AS, Tershner S, Fuller SA, Stein EA . (1997): Cannabinoid induced alterations in regional cerebral blood flow in therat. Pharmacol Biochem Behav 57: 625–631
Busija DW, Heistad DD . (1984): Factors involved in the physiological regulation of the cerebral circulation. Rev Physiol Biochem Pharmacol 101: 161–211
Cador M, Robbins TW, Everitt BJ . (1989): Involvement of the amygdala in stimulus-reward associations: interactions with the ventral striatum. Neuroscience 30: 77–86
Chait LD, Pierri J . (1992): Effects of smoked marijuana on human performance: A critical review. In Murphy L, Bartke A (eds.), Marijuana/Cannabinoids: Neurobiology and Neurophysiology. Boca Raton, CRC Press, pp 387–423
Chen J, Marmus R, Pulles A, Paredes W, Gardner EL . (1993): Ventral tegmental microinjection of Δ9-tetrahydrocannabinol enhances ventral tegmental somatodendritic dopamine levels but not forebrain dopamine levels: Evidence for local neural action by marijuana's psychoactive ingredient. Brain Res 621: 65–70
Chen J, Parades W, Li J, Smith D, Lowinson J, Gardner EL . (1990): Δ9-Tetrahydrocannabinol enhances presynaptic dompamine efflux in medial prefrontal cortex. Eur J Pharmacol 190: 259–262
Childers SR, Sexton T, Roy MB . (1994): Effects of anandamide on cannabinoid receptors in rat brain membranes. Biochem Pharmacol 47: 711–715
Compton DR, Harris LS, Lichtman AH, Martin BR . (1996): Marihuana. In Schuster CR, Kuhar MJ (eds), Pharmacological Aspects of Drug Dependence: Toward an Integrated Neurobehavioral Approach, Handbook of Experimental Pharmacology. pp, 83–158
Crawley JN, Corwin RL, Robinson JK, Felder CC, Devane WA, Axelrod J . (1993): Anandamide, an endogenous ligand of the cannabinoid receptor, induces hypomotility and hypothermia in vivo in rodents. Pharmacol Biochem Behav 46: 967–972
Deutsch DG, Chin SA . (1993): Enzymatic synthesis and degradation of anandamide, a cannabinoid receptor agonist. Biochem Pharmacol 46: 791–796
Devane W, Axelrod J . (1994): Enzymatic synthesis of anandamide, and endogenous ligand for the cannabinoid receptor by brain membranes. Proc Natl Acad Sci USA 91: 6698–6701
Devane WA, Dysarz FA, Johnson MR, Melvin LS, Howlett AC . (1988): Determination and characterization of a cannabinoid receptor in rat brain. Mol Pharmacol 34: 605–613
Devane WA, Hanus L, Breuer A, Pertwee RG, Stevenson LA, Griffin G, Gibson D, Mandelbaum A, Etinger A, Mechoulam R . (1992): Isolation and structure of a brain consistent that binds to the cannabinoid receptor. Science 258: 1946–1949
Dewey WL . (1986): Cannabinoid pharmacology. Pharmacol Rev 38: 151–178
Ferri S, Costa G, Murari G, Panico AM, Rapisaarda E, Speroni E, Arrigo-Reina R . (1981): Investigations on behavioral effects of an extract of Cannabis sativa L in the rat. Psychopharmacology 75: 144
Finch DA . (1993): Hippocampal, subicular, and entorhinal afferents and synaptic integration in rodent cingulate cortex. In Vogt BA, Gabriel M (eds), Neurobiology of the Cingulate Cortex and Limbic Thalamus. Boston, Birkhäuser, pp, 224–248
Fride E, Mechoulam R . (1993): Pharmacological activity of the cannabinoid receptor agonist, anandamide, a brain constituent. Eur J Pharmacol 231: 313–314
Gardner EL, Lowinson JH . (1991): Marijuana's interaction with brain reward systems: Update 1991. Pharmacol Biochem Behav 40: 571–580
Gardner EL, Parades W, Smith D, Donner A, Milling C, Cohen D, Morrison D . (1988): Facilitation of brain stimulation reward by Δ9-tetrahydrocannabinol. Psychopharmacology 96: 142–144
Gloor P . (1975): Electrophysiological studies of the amygdala (stimulation and recording) : Their possible contributions to the understanding of neural mechanisms of aggression. In Fields WS, Sweet WH (eds), Neural Basis of Violence and Aggression. St. Louis, MO, Warren O. Green, pp, 5–40
Gough AL, Olley JE . (1978): Catalepsy induced by intrastriatal injections of delta-9-THC and 11 hydroxy-delta-9-THC in the rat. Neuropharmacology 17: 137–144
Herkenham M, Groen BGS, Lynn AB, Decosta BR, Richfield EK . (1991a): Neuronal localization of cannabinoid receptors and second messengers in mutant mouse cerebellum. Brain Res 552: 301–310
Herkenham M, Lynn AB, Johnson MR, Melvin LS, de Costa BR, Rice KC . (1991b): Characterization and localization of cannabinoid receptors in rat brain: A quantitative in vitro autoradiographic study. J Neurosci 11: 563–583
Heyser CJ, Hampson RE, Deadwyler SA . (1993): Effects of Δ9-tetrahydrocannabinol on delayed match to sample performance in rats: Alterations in short-term memory associated with changes in task specific firing of hippocampal cells. J Pharmacol Exp Ther 264: 294–307
Hillard CJ, Edgemond WS, Campbell WB . (1995): Characterization of ligand binding to the cannabinoid receptor of rat brain membranes using an novel method: Application to anandamide. J Neurochem 64: 677–683
Howlett AC, Bidaut-Russell M, Devane WA, Melvin LS, Johnson MR, Herkenham M . (1990): The cannabinoid receptor: Biochemical, anatomical, and behavioral characterization. TINS 13: 420–423
Jansen EM, Haycock DA, Ward SJ, Seybold VS . (1992): Distribution of cannabinoid receptors in rat brain determined with aminoalkylindoles. Brain Res 575: 93–102
Jay TM, Lucignani G, Crane AM, Jehle J, Sokoloff L . (1988): Measurement of local cerebral blood flow with [14C]iodoantipyrine in the mouse. J Cereb Blood Flow Metab 8: 121–129
Kelley AE, Domesick VB, Nauta WJH . (1982): The amygdalostriatal projection in the rat—An anatomical study by anterograde and retrograde methods. Neuroscience 7: 615–630
Krettek JE, Price JL . (1977): Projections from the amygdaloid complex to the cerebral cortex and thalamus in the rat and cat. J Comp Neurol 172: 687–722
Lichtman AH, Martin BR . (1991): Spinal and supraspinal mechanisms of cannabinoid-induced antinociception. J Pharmacol Exp Ther 258: 517–523
Lichtman AH, Dimen KR, Martin BR . (1995): Systemic or intrahippocampal cannabinoid administration impairs spatial memory in rats. Psychopharmacology 119: 282–290
Mackie K, Hille B . (1992): Cannabinoids inhibit N-type calcium channels in neuroblastoma-glioma cells. Proc Natl Acad Sci USA 89: 3825–3829
Margulies JE, Hammer RP Jr. . (1991): Δ9-Tetrahydrocannabinol alters cerebral metabolism in a biphasic, dose-dependent manner in rat brain. Eur J Pharmacol 202: 373–378
Martin WJ, Lai NK, Patrick SL, Tsou K, Walker JM . (1993): Antinociceptive actions of cannabinoids following intraventricular administration in rats. Brain Res 629: 300–304
Mathew RJ, Wilson WH . (1993): Acute changes in cerebral blood flow after smoking marijuana. Life Sci 52: 757–767
Mathew RJ, Wilson WH, Tant SR . (1989): Acute changes in cerebral blood flow associated with marijuana smoking. Acta Psychiat Scand 79: 108–128
Mathew RJ, Wilson WH, Humphreys DF, Lowe JV, Wiethe KE . (1992): Regional cerebral blood flow after marijuana smoking. J Cereb Blood Flow Metab 12: 750–758
Matsuda LA, Bonner TI, Lolait SJ . (1993): Localization of cannabinoid receptor mRNA in rat brain. J Comp Neurol 327: 535–550
Mogenson GJ, Jones DL, Yim CY . (1980): From motivation to action: Functional interface between the limbic system and the motor system. Prog Neurobiol 14: 69–97
Moskowitz H . (1985): Marihuana and driving. Accid Annal Prevent 17: 323–345
Noyes JR, Brunk SF, Avery DH, Canter A . (1975): The analgesic properties of Δ9-tetrahydrocannabinol and codeine. Clin Pharmacol Ther 18: 84–89
O'Keefe J . (1979): A review of hippocampal place cells. Prog Neurobiol 13: 419–439
Paxinos G, Watson C . (1986): The Rat Brain in Stereotaxic Coordinates, 4th ed. New York, Academic Press
Pertwee RG . (1972): The ring test: A quantitative method for assessing the “cataleptic” effects of cannabis in mice. Brit J Pharmacol 46: 753–763
Pertwee RG, Wickens AP . (1991): Enhancement by chlordiazepoxide of catalepsy induced in rats by intravenous or intrapallidal injections of enantiomeric cannabinoids. Neuropharmacology 30: 237–244
Pertwee RG . (1988): The central neuropharmacology of psychotropic cannabinoids. Pharmac Ther 36: 189–261
Porrino LJ, Domer FR, Crane AM, Sokoloff L . (1988): Selective alterations in cerebral metabolism within the mesocorticolimbic dopaminergic system produced by acute cocaine administration in the rat. Neuropsychopharmacology 1: 109–118
Sakurada O, Kennedy C, Jehle J, Brown JD, Carbin GL, Sokoloff L . (1978): Measurement of local cerebral blood flow with iodo-[14C]antipyrine. Am J Physiol 234: H59–H66
Schwartz RH . (1993): Chronic marihuana smoking and short-term memory impairment. In Nahas GC, Latour C, (eds), Cannabis: Physiopathology, Epidemiology, Detection. Boca Raton, CRC Press, pp, 61–71
Smith PB, Martin BR . (1992): Spinal mechanisms of Δ9-tetrahydrocannabinol-induced analgesia. Brain Res 578: 8–12
Smith PB, Compton DR, Welch SP, Razdan RK, Mechoulam R, Martin BR . (1994): The pharmacological activity of anandamide, a putative endogenous cannabinoid, in mice. J Pharmacol Exp Ther 270: 219–227
Sokoloff L . (1981): Relationships among local functional activity, energy metabolism, and blood flow in the central nervous system. Fed Proc 40: 2311–2316
Sokoloff L, Reivich M, Kennedy C, Des Rosiers MH, Patlak CS, Pettigrew KD, Sakurada O, Shinohara M . (1977): The [14C]deoxyglucose method for the measurement of local cerebral glucose utilization: Theory, procedure, and normal values in the conscious and anesthetized albino rat. J Neurochem 28: 897–916
Squire LR . (1987): Memory: Neural organiztion and behavior. In Mountcastle V, Plum F, Geigner SR (eds), Handbook of Physiology—The Nervous System. Bethesda, MD, American Physiological Society
Stein EA, Fuller SA . (1992): Selective effects of cocaine on regional cerebral blood flow in the rat. J Pharmacol Exper Therapeu 262: 327–334
Stein EA, Fuller SA, Edgemond WS, Campbell WB . (1996): Physiological and behavioral effects of the endogenous cannabinoid, arachidonylethanolamide (anandamide), in the rat. Brit J Pharmacol 119: 107–114
Swanson LW . (1981): A direct projection from Amman's horn to prefrontal cortex in the rat. Brain Res 217: 150–154
Thomas BF, Wei X, Martin BR . (1992): Characterization and autoradiographic localization of the cannabinoid binding site in rat brain using [3H]11-OH-Δ9-THC-DMH. J Pharmacol Exp Ther 263: 1383–1390
Trusk TC, Stein EA . (1987): Effect of intravenous heroin and naloxone on regional cerebral blood flow in the conscious rat. Brain Res 406: 238–245
Varga K, Lake KJ, Martin BR, Kunos G . (1995): Novel antagonist implicates the CB1 cannabinoid receptor in the hypotensive action of anandamide. Eur J Pharmacol 278: 279–283
Vogel Z, Barg J, Levy R, Saya D, Heldman E, Mechoulam R . (1993): Anandamide, a brain endogenous compound, interacts specifically with cannabinoid receptors and inhibits adenylate cyclase. J Neurochem 61: 352–355
Volkow ND, Gillespie H, Mullani N, Tancredi L, Grant C, Ivanovic M, Hollister L . (1991): Cerebellar metabolic activation by delta-9-tetrahydrocannabinol in human brain: A study with positron emission tomography and 18F-2-fluoro-2-deoxyglucose. Psychia Res 40: 69–78
Volkow ND, Gillespie H, Mullani N, Tancredi L, Grant C, Valentine A, Hollister L . (1996): Brain glucose metabolism in chronic marijuana users at baseline and during marijuana intoxication. Psychia Res 67: 29–38
Welch SP, Thomas C, Patrick GS . (1994): Modulation of cannabinoid-induced antiociception following intracerebroventricular versus intrathecal administration to mice: possible mechanisms for interaction with morphine. J Pharmacol Exp Ther 272: 310–331
Wise RA, Rompre P-P . (1989): Brain dopamine and reward. Ann Rev Psychol 40: 191–225
Acknowledgements
Supported in part by grants from the Peters Foundation, National Heart Lung and Blood Institute (HL37981 and HL51055), and National Institutes on Drug Abuse (DA09465 and DA09155).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Stein, E., Fuller, S., Edgemond, W. et al. Selective Effects of the Endogenous Cannabinoid Arachidonylethanolamide (Anandamide) on Regional Cerebral Blood Flow in the Rat. Neuropsychopharmacol 19, 481–491 (1998). https://doi.org/10.1016/S0893-133X(98)00043-8
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1016/S0893-133X(98)00043-8