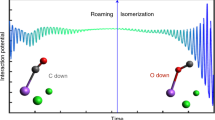
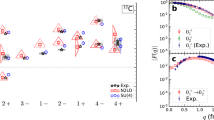
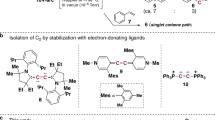
Abstract
THE direct experimental values of the sublimation heat L of carbon obtained by several authors1 lie between 139 and 177 kcal. An accurate value can be deduced from the energy of dissociation DCO of CO into normal atoms, since the well-known relations (at 0°K.): lead to L = DCO 86.3 ± 0.2 kcal. (86.3 kcal. = 3.74 v.e.)(1)
Similar content being viewed by others
Article PDF
References
H. Kohn and M. Guckel, Z. Phys., 27, 305 ; 1924. A. L. Marshall, and F. J. Norton, J. Amer. Chem. Soc., 55, 431 ; 1933.
D. Coster and F. Brons, Physica, 1, 155 ; 1, 634 ; 1934. D. N. Read, Phys. Rev., 45, 752 ; 1934. R. Schmid and L. Gerö, Z. Phys., 93, 656 ; 1935.
L. Pauling and J. Sherman, J. Chem. Phys., 1, 606 ; 1933. C. T. Zahn, J. Chem. Phys., 2, 671 ; 1934. W. Lasareff, J. Phys. Chem. (in the press) and Physica (in the press). P. Goldfinger, W. Lasareff and M. Letort, C.R., 200, 1593; 1935.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
GOLDFINGER, P., LASAREFF, W. Dissociation Energy of the CO Molecule and the Sublimation Heat of Carbon. Nature 135, 1077 (1935). https://doi.org/10.1038/1351077a0
Issue Date:
DOI: https://doi.org/10.1038/1351077a0
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.