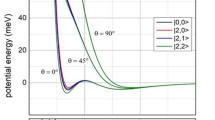
Abstract
The desire to better understand the quantum nature of isomerization led to recent experimental observations of the vibrationally induced isomerization of OC–NaCl(100) to CO–NaCl(100). To investigate the mechanism of this isomerization, we performed dynamics calculations using finite (CO–NaCl)n cluster models. We constructed new potential energy surfaces for CO–NaCl and CO–CO interactions using high-level ab initio data and report key properties of the bare CO–NaCl potential energy surface, which show much in common with the experiment. We investigated the isomerization dynamics using several cluster models and, in all cases, isomerization was seen for highly excited CO vibrational states, in agreement with experiments. A detailed examination of the reaction trajectories indicates that isomerization occurs when the distance between CO and NaCl is larger than the distance at the conventional isomerization saddle point, which is a strong indicator of ‘roaming’.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
All the data shown here are available in the Supplementary Information. Source data are provided with this paper.
Code availability
The potentials reported in the paper are available in compressed folders as Supplementary Information.
References
Bowman, J. M., Gazdy, B., Bentley, J. A., Lee, T. J. & Dateo, C. E. Ab initio calculation of a global potential, vibrational energies, and wave functions for HCN/HNC, and a simulation of the \(\tilde A\)−\(\tilde A\) emission spectrum. J. Chem. Phys. 99, 308–323 (1993).
Bowman, J. M. Beyond platonic molecules. Science 290, 724–725 (2000).
Zou, S., Bowman, J. M. & Brown, A. Full-dimensionality quantum calculations of acetylene vinylidene isomerization. J. Chem. Phys. 118, 10012–10023 (2003).
DeVine, J. A. et al. Encoding of vinylidene isomerization in its anion photoelectron spectrum. Science 358, 336–339 (2017).
Lau, J. A. et al. Observation of an isomerizing double-well quantum system in the condensed phase. Science 367, 175–178 (2020).
Chen, L. et al. The Sommerfeld ground-wave limit for a molecule adsorbed at a surface. Science 363, 158–161 (2019).
Vogt, J. & Vogt, B. The structure of carbon monoxide adsorbed on the NaCl(100) surface: a combined LEED and DFT-D/vdW-DF study. J. Chem. Phys. 141, 214708 (2014).
Chen, J., Li, J., Bowman, J. M. & Guo, H. Energy transfer between vibrationally excited carbon monoxide based on a highly accurate six-dimensional potential energy surface. J. Chem. Phys. 153, 054310 (2020).
Hoang, P. N. M., Picaud, S., Girardet, C. & Meredith, A. W. Structure of CO monolayer adsorbed on NaCl(100) from molecular dynamics. J. Chem. Phys. 105, 8453–8462 (1996).
Meredith, A. W. & Stone, A. J. A perturbation theory study of adlayer CO on NaCl(100). J. Chem. Phys. 104, 3058–3070 (1996).
Corcelli, S. A. & Tully, J. C. Vibrational energy pooling in CO on NaCl(100): methods. J. Chem. Phys. 116, 8079–8092 (2002).
Boney, E. T. D. & Marcus, R. A. On the infrared fluorescence of monolayer 13CO:NaCl(100). J. Chem. Phys. 139, 184712 (2013).
Boese, A. D. & Saalfrank, P. CO molecules on a NaCl(100) surface: structures, energetics, and vibrational Davydov splittings at various coverages. J. Phys. Chem. C 120, 12637–12653 (2016).
Chen, J., Hariharan, S., Meyer, J. & Guo, H. Potential energy landscape of CO adsorbates on NaCl(100) and implications in isomerization of vibrationally excited CO. J. Phys. Chem. C 124, 19146–19156 (2020).
Townsend, D. et al. The roaming atom: straying from the reaction path in formaldehyde decomposition. Science 306, 1158–1161 (2004).
Suits, A. G. Roaming atoms and radicals: a new mechanism in molecular dissociation. Acc. Chem. Res. 41, 873–881 (2008).
Bowman, J. M. & Shepler, B. C. Roaming radicals. Ann. Rev. Phys. Chem. 62, 531–553 (2011).
Homayoon, Z. & Bowman, J. M. Quasiclassical trajectory study of CH3NO2 decomposition via roaming mediated isomerization using a global potential energy surface. J. Phys. Chem. A 117, 11665–11672 (2013).
Zhu, R. & Lin, M. CH3NO2 decomposition/isomerization mechanism and product branching ratios: an ab initio chemical kinetic study. Chem. Phys. Lett. 478, 11–16 (2009).
Dey, A. et al. Photodissociation dynamics of nitromethane and methyl nitrite by infrared multiphoton dissociation imaging with quasiclassical trajectory calculations: signatures of the roaming pathway. J. Chem. Phys. 140, 054305 (2014).
Wodtke, A. M., Hintsa, E. J. & Lee, Y. T. Infrared multiphoton dissociation of three nitroalkanes. J. Phys. Chem. 90, 3549–3558 (1986).
Acknowledgements
J.M.B. and H.G. thank the Alexander von Humboldt Foundation for Humboldt Research Awards. In addition, J.M.B. thanks NASA (80NSSC20K0360) for financial support and H.G. thanks the National Science Foundation (CHE-1462109 and CHE-1951328) for financial support. We thank J. Lau and A. Wodtke for extensive discussions.
Author information
Authors and Affiliations
Contributions
A.N. performed the dynamics calculations, developed the CO–NaCl potential and cluster potential, refitted the CO–CO potential and helped write the paper and prepared most of the figures. P.Z. performed DFT calculations of energies of the CO–NaCl stationary points. J.C. performed electronic structure calculations of the CO–CO interaction. H.G. helped conceive the research, helped writing the paper and supervised the creation of the CO–CO electronic energy dataset. J.M.B. conceived the research, wrote the paper and supervised the dynamics calculations.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature Chemistry thanks Jochen Vogt and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–14, and Tables 1–9.
Supplementary Video 1
QCT Trajectory Animation.
Supplementary Software 1
All three PES Software. ‘CO_CO_Int’ folder contains CO-CO interaction PES; ‘CO_MRCI’ folder contains isolated CO PES; and ‘NaCl_CO_Int’ folder contains NaCl-CO interaction PES.
Supplementary Data 1
Source data for the figures within the Supplementary Information file.
Source data
Source Data Fig. 3
Source data for Figure 3.
Source Data Fig. 4
Source data for Figure 4.
Source Data Fig. 5
Source data for Figure 5 .
Source Data Fig. 6
Source data for Figure 6.
Rights and permissions
About this article
Cite this article
Nandi, A., Zhang, P., Chen, J. et al. Quasiclassical simulations based on cluster models reveal vibration-facilitated roaming in the isomerization of CO adsorbed on NaCl. Nat. Chem. 13, 249–254 (2021). https://doi.org/10.1038/s41557-020-00612-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41557-020-00612-y
This article is cited by
-
Deep learning study of tyrosine reveals that roaming can lead to photodamage
Nature Chemistry (2022)