Abstract
Bupropion (BUP) is a dopamine (DA) and norepinephrine (NE) reuptake inhibitor that causes mild weight loss in obese adults. Subchronic (7 day) coadministration of selective DA and NE reuptake inhibitors also causes weight loss in mice. Because weight loss was not associated with decreased caloric intake, subchronic BUP might cause weight loss through increased energy expenditure. Acute studies demonstrate that BUP or DA+NE reuptake inhibitors cause transient hypophagia and increased locomotion; though the effects on temperature are inconsistent. Because subchronic DA+NE reuptake inhibition does not affect appetite, there is clearly a difference between the acute and subchronic effects of DA+NE reuptake inhibitors; however the effects of chronic (or subchronic) BUP on energy balance have never been directly studied in an animal model. Therefore, the acute and subchronic effects of BUP or selective DA and NE reuptake inhibitors on food intake, body weight, locomotor activity, and interscapular temperature were determined in mice. Generally, selective inhibition of DA reuptake (by GBR12783) increased activity while selective inhibition of NE reuptake (by nisoxetine, NIS) decreased activity and temperature. BUP increased activity and temperature but subchronic BUP did not significantly reduce body weight due to a compensatory increase in food intake. Subchronic DA+NE reuptake inhibitor coadministration mimicked the effect of BUP on activity and temperature, but caused weight loss because daily food intake was not increased. The results of this study suggest that the mild weight loss effect of BUP in humans may be due to increased locomotion or heat production. More importantly, inhibition of DA+NE reuptake (with GBR+NIS) increased energy expenditure without a compensatory increase in food intake, supporting a role for novel combination catecholamine reuptake inhibitors in pharmacotherapy for obesity.
Similar content being viewed by others
INTRODUCTION
Bupropion (BUP) is currently approved for the treatment of depression (Wellbutrin®) and as an aid in smoking cessation (Zyban®) (Ascher et al, 1995; Foley et al, 2006). Originally associated with a ‘lack of weight gain’, BUP was recently shown to cause mild (5%) weight loss in clinical trials with obese human subjects (Gadde and Xiong, 2007). Although BUP is undergoing further clinical studies as a pharmacotherapy for obesity, there are no controlled comprehensive animal studies that assess how chronic BUP affects energy balance.
Like many antidepressants and the only centrally acting, FDA-approved long-term obesity treatment, sibutramine (Meridia®), BUP inhibits biogenic amine plasma membrane reuptake transporters (Ascher et al, 1995; Stahl et al, 2004; Kaplan, 2005). By selectively inhibiting the reuptake transporters for dopamine (DA) and norepinephrine (NE), BUP impinges on the primary means for clearance of extracellular catecholamines (Hoffman et al, 1998). Through blockade of the DA and NE transporters, BUP causes acute increases in interstitial catecholamine concentrations and increased feedback inhibition of presynaptic neurons (Ascher et al, 1995; Dong and Blier, 2001).
BUP reduces food intake when administered acutely to rodents (Zarrindast and Hosseini-Nia, 1988; Billes and Cowley, 2007). Because acute coadministration of selective DA+NE reuptake inhibitors produces an additive inhibitory effect on food intake in mice, both catecholamines probably contribute to the acute hypophagic effect of BUP. Both DA and NE also appear to contribute to the weight loss effect of BUP that has been documented in humans, as subchronic (7 day) coadministration of DA+NE reuptake inhibitors also causes weight loss in mice (Billes and Cowley, 2007). Interestingly, weight loss caused by subchronic DA+NE reuptake inhibition in mice occurred in the absence of a reduction in food intake, which is also similar to the observation that chronic BUP administration does not affect caloric intake in humans (Griffith et al, 1983; Harto-Truax et al, 1983). This suggests that catecholamine reuptake inhibition may cause weight loss by increasing energy expenditure.
Current data in rodents indicate that acute BUP may increase energy expenditure by increasing temperature (Liu et al, 2002, 2004). Because reports on the acute effects of BUP on temperature are inconsistent, further research is needed to determine if increased thermogenesis might also contribute to increased energy expenditure by BUP (Zarrindast and Abolfathi-Araghi, 1992; Liu et al, 2002; Hasegawa et al, 2005). We and others have shown that acute peripheral BUP also dose dependently stimulates locomotor activity in rodents, an effect that is consistent with inhibition of the DA transporter (Soroko et al, 1977; Cooper et al, 1980; Nielsen et al, 1986; Zarrindast and Hosseini-Nia, 1988; Vassout et al, 1993; Redolat et al, 2005; Mitchell et al, 2006).
Obesity is generally regarded as a chronic disease requiring continuous intervention to maintain low body weight (Appolinario et al, 2004; Ioannides-Demos et al, 2005). Chronic (5–21 days) treatment with drugs like BUP can affect receptor expression, intracellular signaling mechanisms, and transporter expression and activity (Frazer and Benmansour, 2002). Thus, chronic BUP treatment can result in behavioral tolerance and/or sensitization such that the acute effects of BUP on energy balance may differ from BUP's chronic effects. Studies that address how chronic DA+NE reuptake inhibition affect energy intake and expenditure are necessary to determine how long-term drug administration affects energy balance.
There is a conspicuous absence of data on the comprehensive effects of chronic BUP treatment on energy balance in animal models. It is also unclear how DA and NE contribute to the possible metabolic effects of BUP, as no studies have examined how chronic administration of selective DA or NE reuptake inhibitors affects energy expenditure. Studies comparing the effects of BUP with that of selective DA or NE reuptake inhibitors on multiple measures of energy balance (such as food intake, locomotor activity, thermogenesis, and body weight) are necessary for a complete analysis of the effects of drugs like BUP on energy balance. The purpose of the present study was to examine the acute and chronic effects of DA+NE reuptake inhibition on energy balance in mice, particularly to shed light on the mechanism of weight loss by drugs like BUP. To this aim, we first investigated the effects of acute administration of BUP or selective DA and NE reuptake inhibitors on locomotor activity and interscapular temperature. We then investigated the effects of subchronic (7 day) BUP or selective DA and NE reuptake inhibitors on locomotor activity, interscapular temperature, daily food intake, and daily body weight.
MATERIALS AND METHODS
Animal Care and Housing
All animal procedures were approved by the Oregon National Primate Research Center Institutional Animal Care and Use Committee. Adult male C57Bl/6J mice (Jackson Labs, Bar Harbor, ME) were individually housed under a 12 h light/dark cycle and constant temperature (22±1°C). Food and water were available ad libitum, unless specified otherwise. Mice were maintained on standard chow (Purina Lab Chow, no. 5001). All surgical procedures were performed under isoflurane anesthesia using aseptic surgical procedure.
Drugs
Drugs were prepared fresh on day of use. For intraperitoneal (i.p.) administration, BUP (Sigma, St Louis, MO) and nisoxetine (NIS; Tocris, Ellisville, MO) were dissolved in sterile nonpyrogenic 0.9% NaCl. GBR12783 (GBR; Tocris) and GBR+NIS were dissolved in 10% dimethyl sulfoxide and saline. All acutely administered drugs were given i.p. in a volume of 0.1±0.02 ml (according to body weight). Control mice received vehicle in a corresponding volume. For subchronic administration (via Alzet® minipumps), NIS was dissolved in 10% dimethyl sulfoxide and sterile nonpyrogenic saline. BUP and GBR were dissolved in 50% dimethyl sulfoxide and 50% sterile H2O. Previously published data were used to determine drug doses that would have a moderate effect on energy balance (Billes and Cowley, 2007).
Telemetric Transponder Implantation and Locomotor Activity and Temperature Measurement
Remote biotelemetry was performed using precalibrated sensitive transmitters (PDT-4000 G2 E-Mitter® sensors, Mini Mitter Company, Sun River, OR). Under isoflurane anesthesia, E-Mitters were implanted beneath the interscapular brown adipose tissue (IBAT) pad between the scapulae, and wounds were closed with sutures. Mice were allowed 1-week recovery before studies commenced. Signals emitted by the E-Mitter transmitters were detected by a receiver positioned underneath the animal's home cage and converted into activity counts (arbitrary units) by VitalView® software (Mini Mitter) (Harkin et al, 2002). Locomotor activity counts are a relative measure of gross motor activity. For all experiments, activity counts and interscapular temperature measurements were taken every 6 min.
Acute Drug Administration Studies
Mice were habituated to the behavioral testing paradigm by daily i.p. injection of 0.1 ml sterile saline and 16 h (overnight) fasting every third day for at least 2 weeks. Then mice were implanted with interscapular PDT-4000 E-Mitter sensors, allowed to recover, and habituated for 2 weeks before beginning feeding studies. All mice were assigned to treatment groups balanced for body weight. On the morning of testing, 16 h fasted mice received i.p. injection of freshly prepared drug or vehicle, were returned to their cage, and given six pre-weighed food pellets. As a positive control for drug efficacy, food was weighed at 1, 2, and 4 h post injection.
Subchronic Drug Administration Studies
Because chronic drug administration studies usually encompass 2–3 weeks of drug treatment, we have termed our abbreviated 7-day chronic study as subchronic. We were limited to 7 days by drug solubility; extending the study further would require changing minipumps after each week of treatment, potentially confounding results. Because of these technical complications, we chose to conduct a week-long subchronic study. Mice were implanted with interscapular PDT-4000 G2 E-Mitter® sensors and allowed to recover for 1 week. They were then divided into weight-matched groups and implanted with subcutaneous Alzet mini-osmotic pumps (Durect Corporation, Cupertino, CA) that delivered drug for 7 days. Osmotic pumps were implanted via a small incision in the skin 1 cm caudal to the scapulae and slightly lateral to the vertebrae. The incision was closed with wound clips and mice were monitored daily. Mice in the subchronic BUP study were implanted with one minipump (model no. 2001) that contained either BUP (10 mg/kg/h) or vehicle. To minimize possible drug interactions, mice in the GBR+NIS coadministration study received two minipumps that contained either NIS (1.5 mg/kg/h) or vehicle (model no. 1007D) and GBR (2.5 mg/kg/h) or vehicle (model no. 2001). Food intake (corrected for spillage) and body weights were recorded daily at 1100 hours. The average initial body weight for animals in subchronic BUP studies was 27.0 g. The average initial body weight for animals in subchronic GBR+NIS studies was 24.5 g.
Statistical Analysis
Data sets were analyzed using Prism Software (Graph-Pad Software, San Diego, CA). For locomotor activity, area under the curve (AUC) was calculated by trapezoid analysis. For acute studies, one-way analysis of variance (ANOVA) and Bonferroni's multiple comparison post-tests were used to determine significant differences in AUC locomotor activity, average interscapular temperature, and food intake between vehicle- and drug-treated animals at different post-injection time intervals. For studies in Figures 1 and 2, vehicle-treated animals were pooled within their respective experiments. For subchronic drug administration studies, average daily locomotor activity was calculated as the average of AUC (counts/12 h) for either light or dark phase on days 2–7. Average interscapular temperature was calculated as the average temperature during either the light or dark phase for days 2–7. Student's t-test was used to determine significant differences in average activity or temperature between vehicle- and BUP-treated animals in either the light or dark phase. One-way ANOVA followed by Bonferroni's multiple comparison post-tests was used to determine significant differences in average light- or dark-phase activity or temperature between vehicle-, NIS-, GBR-, or GBR+NIS-treated animals. Two-way ANOVA followed by Bonferroni's post-tests was used to compare differences in average daily AUC locomotor activity, average temperature, daily food intake, and body weight between vehicle- and drug-treated animals across time (days 1–7). Data are expressed as mean and the standard error of the mean (SEM) except where noted. P-values of <0.05, <0.01, and <0.001 were considered significant.
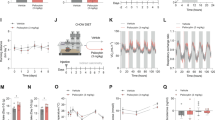
Effect of acute bupropion (BUP) on interscapular temperature. Overnight food-deprived mice were treated with BUP (10, 20, or 40 mg/kg i.p.) or vehicle and food was returned to the cage. (a) Time course for interscapular temperature for BUP-treated mice and control. (b) BUP (20 and 40 mg/kg) significantly reduced 1 h temperature compared to vehicle (*P<0.05, ***P<0.001 for treatment group vs control).
Effect of acute inhibition of DA and NE reuptake on locomotor activity and interscapular temperature. Overnight food-deprived mice were treated with nisoxetine (NIS, 4 mg/kg), GBR12783 (GBR, 7.5 mg/kg), NIS (4 mg/kg)+GBR (7.5 mg/kg) or vehicle and food was returned to the cage. (a) Time course for activity following drug or vehicle injection. (b) AUC for locomotor activity from 0 to 1 h post injection. (c) Time course for interscapular temperature. (d) Average interscapular temperature from 0 to 1 h post injection for all treatment groups (*P<0.05, **P<0.01, ***P<0.001 for treatment group vs control).
RESULTS
Acute BUP Caused Mild Transient Hypothermia Followed by Mild Hyperthermia
We have previously shown that acute BUP causes a dose-dependent and transient increase in locomotor activity in mice that is concurrent with a transient inhibitory effect on food intake up to 1 h post injection (Billes and Cowley, 2007). The time course for average interscapular temperature following 10, 20, and 40 mg/kg BUP is shown in Figure 1a (n=8, animals/treatment group; n=22, vehicle-treated animals). BUP had a significant effect on average interscapular temperature during the 60 min following injection (F(3, 446)=16.40, P<0.001). BUP (20 and 40 mg/kg) significantly decreased average interscapular temperature compared to control (Figure 1b). However, between 1 and 2 h post injection, 10, 20, and 40 mg/kg BUP prevented the decrease in average interscapular temperature in control animals by 0.4, 0.6, and 0.64°C (P<0.01), respectively (F(3, 446)=21.96, P<0.001). Only 20 mg/kg increased average temperature 2–4 h post injection by 0.3°C (F(3, 916)=5.22, P=0.0014; 20 mg/kg, P<0.01).
Acute Coadministration of Selective DA and NE Reuptake Inhibitors Increased Activity and Caused Acute Mild Hypothermia Followed by Hyperthermia
As has been previously reported, NIS (4 mg/kg), GBR (7.5 mg/kg), and NIS (4 mg/kg)+GBR (7.5 mg/kg) all significantly reduced food intake at 1 h post injection (F(3, 32)=17.53, P<0.001), but not at any later time points (data not shown). There was a significant effect of treatment on locomotor activity at 1 h post injection (F(3, 32)=10.29, P<0.001; n=5–7, animals/treatment group; n=18, vehicle-treated animals). Inhibition of DA reuptake with GBR and GBR+NIS significantly increased activity at 1 h post injection (Figure 2a and b). GBR also significantly increased locomotor activity from 1 to 2 h post injection (F(3, 32)=10.24, P<0.001) (GBR; P<0.01) data not shown). There was no significant effect of any treatment on locomotor activity from 2 to 4 h post injection.
There was a significant effect of treatment on average interscapular temperature at 1 h post injection (F(3, 356)=9.40, P<0.001), 2 h post injection (F(3, 356)=21.64, P<0.001), and 4 h post injection (F(3, 716)=15.84, P<0.001). Inhibition of NE reuptake by both NIS and GBR+NIS significantly decreased average interscapular temperature from control between 0 and 1 h post injection (Figure 2d). However, between 1 and 2 h post injection, only NIS-treated animals were hypothermic by 0.36°C compared to control animals (P<0.05; data not shown). Between 1–2 and 2–4 h post injection, DA reuptake inhibition by GBR or GBR+NIS increased average temperature by 0.76 and 0.44°C at 2 h, and 0.64 and 0.5°C at 4 h (P<0.001; data not shown).
Subchronic DA+NE Reuptake Inhibition by BUP Increased Activity and Temperature
Average hourly light- and dark (shaded)-phase activity from day 3 is presented in Figure 3a to illustrate the effect of subchronic BUP infusion on daily locomotor activity. To better separate the acute and chronic effects of BUP, average daily locomotor activity or interscapular temperature was calculated as the average of days 2–7. On days 2–7, BUP significantly increased average light- (t(64)=3.99, P=0.0002) and dark-phase activity (t(64)=2.41, P=0.019) (Figure 3b). On day 1 of infusion, BUP (10 mg/kg/h; n=5) caused an acute threefold increase in light-phase activity compared to vehicle (n=4) (Figure 3c). BUP had a significant main effect on locomotor activity during the light phase (F(1, 42)=20.46, P<0.0001) (Figure 3c) but not the dark phase (Figure 3d). Overall, BUP treatment caused a 20% increase in cumulative locomotor activity at day 7 (t(22)=9.419, P<0.0001) (data not shown).
Subchronic bupropion (BUP) infusion increases locomotor activity and interscapular temperature. Adult mice were implanted with mini-osmotic pumps delivering either vehicle or BUP (10 mg/kg/h) for 7 days. (a) Representative trace of hourly activity (taken from day 3) for vehicle- and BUP-treated animals. (b) Average of area under the curve (AUC) values for locomotor activity from days 2 to 7 during the 12 h light and 12 h dark phases. (c) AUC of light-phase locomotor activity. (d) AUC of dark-phase locomotor activity. (e) Representative trace of hourly interscapular temperature (taken from day 3) for BUP- or vehicle-treated animals. (f) Average temperature for the 12 h light and 12 h dark phase from days 2 to 7 of infusion. (g) Average daily light-phase temperature. (h) Average daily dark-phase temperature (*P<0.05, **P<0.01, ***P<0.001 for treatment group vs control).
Figure 3e is a representative trace of average hourly temperature of subchronic vehicle- or BUP-treated animals on day 3 of infusion. Compared to vehicle, subchronic BUP administration significantly increased average interscapular temperature during both the light phase by 0.63±0.03°C compared to control (t(64)=6.12, P<0.0001) and dark phase by 0.19±0.04°C compared to control (t(64)=4.56, P<0.0001) (Figure 3f). BUP significantly increased interscapular temperature on days 1, 2, and 3, and had a significant main effect on interscapular temperature during the light phase (F(1, 42)=17.35, P=0.0042) (Figure 3g) but not the dark phase (Figure 3h). BUP infusion did not significantly affect body weight, although there was a trend toward 2–3% weight loss compared to control (Figure 4a). There was no main effect of BUP on daily food intake, however there was a significant increase in food intake in BUP-treated animals on day 5 (Figure 4b). Cumulative food intake at day 7 was increased by 17% in BUP-treated animals (t(52)=3.14, P=0.0028) (data not shown).
Effect of subchronic bupropion (BUP) administration on daily body weight and food intake. Adult mice received either subchronic BUP (10 mg/kg/h) or vehicle infusion via mini-osmotic pump. (a) Effect of BUP on daily body weight. (b) Effect of BUP on daily food intake (*P<0.05 for treatment group vs control).
Subchronic Coadministration of Selective DA and NE Reuptake Inhibitors Increased Activity and Temperature and Caused Weight Loss
Figure 5a is a representative trace of the effect of subchronic selective DA and NE reuptake inhibitors on locomotor activity on day 3 of infusion. During days 2–7 of infusion, there was a significant effect of treatment on average locomotor activity during the light (F(3, 119)=12.71, P<0.0001) and dark phases (F(3, 119)=18.26, P<0.0001) (Figure 5b). Post-tests revealed that GBR (2.5 mg/kg/h; n=3–4) and NIS (1.5 mg/kg/h)+GBR (2.5 mg/kg/h; n=4) significantly increased average locomotor activity from vehicle (n=5) during the light phase. During the dark phase, NIS (1.5 mg/kg/h; n=5) significantly decreased average locomotor activity and GBR significantly increased average locomotor activity. There was a significant main effect of treatment during the light phase on days 1–7 (F(3, 78)=11.16, P<0.0001) (Figure 5c). On day 1, GBR caused a fivefold increase in locomotor activity and GBR+NIS caused a threefold increase in locomotor activity compared to vehicle-treated animals during the light phase. Both treatments also doubled activity on day 2 in the light phase. In the dark phase, there was a significant main effect of treatment (F(3, 78)=9.527, P<0.01); GBR and GBR+NIS increased locomotor activity on day 1 only (Figure 5d). Overall, NIS treatment caused a 29% decrease in cumulative locomotor activity at day 7 and GBR and GBR+NIS caused a 58 and 28% increase in locomotor activity, respectively (F(3, 13)=11.29, P<0.0006) (data not shown).
Effect of subchronic inhibition of DA and NE reuptake on locomotor activity and interscapular temperature. Adult male mice received vehicle or nisoxetine (NIS, 1.5 mg/kg/h) and vehicle or GBR12783 (GBR, 2.5 mg/kg/h) infusion mini-osmotic pump for 7 days. (a) Representative trace of hourly locomotor activity (taken from day 3) during subchronic infusion of NIS, GBR, GBR+NIS, and vehicle-treated animals. SEM not included for clarity. (b) Average area under the curve (AUC) values for locomotor activity from days 2 to 7 of infusion during the 12 h light and 12 h dark phases. (c) AUC of light-phase locomotor activity. (d) AUC of dark-phase locomotor activity. (e) Representative hourly interscapular temperature (taken from day 3) in NIS, GBR, GBR+NIS, and vehicle-treated animals. (f) Average temperature for the 12 h light and 12 h dark phases on days 2–7 of infusion. (g) Average light-phase temperature. (h) Average dark-phase temperature (*P<0.05, **P<0.01, ***P<0.001 for treatment group vs control).
Figure 5e is a representative trace of average interscapular temperature on day 3 of infusion. There was a significant effect of treatment on average interscapular temperature during the light (F(3, 119)=11.26, P<0.0001) and dark phase (F(3, 119)=11.04, P<0.0001) on days 2–7 of infusion (Figure 5f). During the light phase, NIS significantly decreased average interscapular temperature while GBR and GBR+NIS significantly increased average temperature. GBR+NIS caused a 0.38±0.04°C increase in average light-phase temperature. NIS and GBR significantly decreased average interscapular temperature during the dark phase while GBR+NIS had no effect (Figure 5f). There was a significant main effect of treatment on interscapular temperature during the light phase on days 1–7 (F(3, 78)=25.55, P<0.0001) (Figure 5g). On days, 1, 4, and 5, NIS significantly decreased temperature. GBR+NIS significantly increased light-phase temperature on days 2 and 3. There was no effect of treatment during the dark phase on days 1–7 (Figure 5h).
Only coadministration of catecholamine reuptake inhibitors significantly affected body weight; GBR+NIS significantly decreased body weight on days 4–6 of infusion (Figure 6a). There was also a significant effect of treatment on food intake (F(3, 13)=3.47, P=0.048) (Figure 6b). NIS significantly decreased food intake only on day 1 of infusion and GBR significantly increased food intake on day 4 of infusion. Although not statistically significant, GBR caused a 7% decrease in food intake. GBR+NIS and NIS treatment significantly affected cumulative food intake at day 7, causing a 10 and 19% decrease in food intake from control, respectively (F(3, 13)=5.00, P<0.05) (data not shown).
Effect of subchronic DA and NE reuptake inhibition on daily body weight and food intake. Adult mice received subchronic infusion of either nisoxetine (NIS, 1.5 mg/kg/h), GBR12783 (GBR, 2.5 mg/kg/h), NIS (1.5 mg/kg/h)+GBR (2.5 mg/kg/h), or vehicle. (a) Effect of NIS, GBR, GBR+NIS, or vehicle on daily body weight. (b) Effect of NIS, GBR, GBR+NIS, or vehicle on daily food intake (*P<0.05, **P<0.01 for treatment group vs control; +P<0.05 for NIS-treated animals vs control; #P<0.05 for GBR-treated animals vs control).
DISCUSSION
The present study demonstrates that subchronic (7 day) combined DA+NE reuptake inhibition causes weight loss by increasing locomotor activity and interscapular temperature and not by inhibiting food intake. Combined catecholamine reuptake inhibition with either BUP or GBR+NIS increased both activity and temperature, but BUP did not cause significant weight loss owing to a compensatory increase in food intake. Because the effect of combined selective DA+NE reuptake inhibitors on activity and temperature is different from the individual effects of these drugs, elevated energy expenditure is likely the result of an interaction between DA and NE systems. This study also provides new evidence that the weight loss observed with BUP treatment in obese humans is possibly the result of elevated energy expenditure by increased activity and thermogenesis caused by a combined dopaminergic and noradrenergic mechanism.
Body weight is determined by energy intake and energy expenditure. Energy expenditure can be further divided into basal metabolism, mechanical work (locomotor activity), and adaptive thermogenesis (Spiegelman and Flier, 2001). Thus, elevated locomotor activity and interscapular temperature probably caused increased energy expenditure. Pharmacological and genetic studies support this assertion. Elevated activity and temperature have already been demonstrated to account for increased energy expenditure caused by treatment with the weight loss drug sibutramine (Liu et al, 2002; Golozoubova et al, 2006). In mice on a high-fat diet, elevated activity and temperature prevent weight gain. However, mice lacking the melanocortin-4 receptor do not increase activity and temperature in response to a high-fat diet and exhibit accelerated weight gain (Butler et al, 2001). This data set does not prove that the magnitude of the increase in interscapular temperature and locomotor activity regulates energy expenditure enough to produce changes in body weight. However, they provide a persuasive argument that this is the case. Changes in temperature and locomotor activity are in the appropriate qualitative direction, and in the case of BUP were sufficient to produce no net change in body weight, in spite of increased food intake. We cannot discount the formal possibility that increased interscapular temperature could be due to increased local blood flow, but the large increases in locomotor activity observed in this study would certainly cause an increase in energy use.
Remote biotelemetry allows for simultaneous and continuous measurement of locomotor activity and temperature in the animals' home cage, minimizing confounding variables and allowing for temporal data acquisition (Harkin et al, 2002). By using biotelemetry to measure mechanical work and adaptive thermogenesis in mice during both acute and chronic drug treatment, we were able to record the two types of energy expenditure that vary with energy state (Spiegelman and Flier, 2001).
Because E-Mitters were implanted underneath IBAT, the main organ for adaptive thermogenesis in small mammals, temperature fluctuations probably reflect activity of IBAT (Lowell and Spiegelman, 2000; Avram et al, 2005). The idea that BAT may be a viable target for obesity pharmacotherapy has already been proposed. Increasing BAT activity elevates metabolic rate and prevents diet-induced obesity in rodents, whereas genetic mutation and pharmacological inhibition of BAT can cause obesity (Spiegelman and Flier, 2001; Crowley et al, 2002). Because adaptive thermogenesis is believed to contribute to the decreased energy expenditure characteristic of obesity and caloric deficit (dieting), drugs that increase adaptive thermogenesis (by activating BAT) have been suggested as a possible means to achieve safe and sustained energy expenditure (Major et al, 2007).
Acute and chronically administered BUP has repeatedly been demonstrated to dose dependently increase locomotor activity in rodents with similar potency, regardless of species, age, or strain (Soroko et al, 1977; Nielsen et al, 1986; Zarrindast and Hosseini-Nia, 1988; Vassout et al, 1993; Redolat et al, 2005; Mitchell et al, 2006; Billes and Cowley, 2007). The current finding that BUP, DA, and DA+NE reuptake inhibition all transiently increased locomotor activity is consistent with the idea that inhibition of the DA transporter is sufficient to cause a significant short-term increase in locomotion and even reverse the decrease in activity caused by inhibition of NE reuptake. The transient increase in locomotor activity caused by subchronic BUP infusion is identical to the effect caused by subchronic infusion of various selective DA reuptake inhibitors (Izenwasser et al, 1999). A dopaminergic mechanism for increased locomotor activity by BUP is consistent with increased striatal DA concentrations in rats following acute BUP administration (Vassout et al, 1993), the failure of BUP to increase locomotor activity in rats with selective ablation of DA neurons (Cooper et al, 1980), and a recent report by Mitchell et al (2006) demonstrating that acute BUP increases locomotor activity in NE transporter knockout mice. DA reuptake inhibition also accounts for increased locomotion by other mixed monoamine reuptake inhibitors including psychostimulants and the weight loss drug sibutramine (Missale et al, 1998; Izenwasser et al, 1999; Golozoubova et al, 2006; Mitchell et al, 2006). Thus, the finding that BUP increases locomotor activity is widely supported.
Acute combined DA+NE reuptake inhibition, either with BUP or coadministration of selective DA+NE reuptake inhibitors, caused a brief decrease in interscapular temperature. The only other study examining the effects of BUP on temperature in mice reported that acute BUP causes rapid and pronounced hypothermia (Zarrindast and Abolfathi-Araghi, 1992). More recent studies in rats have demonstrated the opposite effect, instead showing that peripheral BUP causes a small increase in colonic and core temperature and also O2 consumption (Liu et al, 2002, 2004; Hasegawa et al, 2005). Many variables may account for the conflicting acute effects of BUP on temperature. Factors such as species (mice vs rats), dose, route of administration, time of day, duration of temperature measurement, location of temperature measurement (core, peripheral, or IBAT), or ambient temperature could have influenced BUP's effects on temperature. Ambient temperature has been demonstrated to influence the effect of cocaine on body temperature (Lomax and Daniel, 1990). Similar to cocaine, BUP has little effect on temperature in humans in a thermoneutral environment, but may increase temperature in a warm environment due to a higher set point for hyperthermia and reduced compensatory heat loss (Griffith et al, 1983; Watson et al, 2005). Finally, because the current acute studies were performed in the light phase (when animals are less active and have lower body temperature) in fasted animals that were subsequently fed, these results may reflect an acute attenuation of the thermic effect of food by BUP, rather than a decrease in steady-state temperature (Lowell and Spiegelman, 2000). In fact, the mild but significant increase in temperature between 1 and 2 h post injection supports the larger collection of evidence that BUP, and perhaps its pharmacologically active metabolites, increase temperature (Ascher et al, 1995; Liu et al, 2002, 2004; Hasegawa et al, 2005).
NE released from sympathetic nerve terminals activates β3-adrenoceptors on brown adipocytes in BAT and leads to lipolysis, increased activity of uncoupling protein-1 (UCP-1), and thermogenesis (Avram et al, 2005; Fan et al, 2005). By blocking NE reuptake in sympathetic nerve terminals, an NE reuptake inhibitor could increase β3-adrenoceptor activation, thereby increasing oxidative phosphorylation and the production of heat (Iversen, 1971). In rats, antagonism of the β3-adrenoceptor attenuates the increased O2 consumption caused by BUP (Liu et al, 2004) and the weight loss drug sibutramine (Connoley et al, 1999), suggesting that BAT activation accounts for a significant proportion of the increased energy expenditure with these drugs. The current finding that selective NE reuptake inhibition decreased interscapular temperature emphasizes that additional dopaminergic (with BUP treatment) or serotonergic (with sibutramine treatment) input is important to maintain increased sympathetic tone and cause thermogenesis.
Because thermogenesis through BAT activation is a metabolic regulatory process that is controlled by the hypothalamus via descending sympathetic fibers (Lowell and Spiegelman, 2000), catecholamine reuptake inhibitors may act both centrally and peripherally to influence interscapular temperature (Wellman, 2005). BUP has been shown to increase DA and NE in hypothalamic nuclei that regulate body temperature such as the preoptic area and anterior hypothalamus (Hasegawa et al, 2005). Catecholamine reuptake inhibitors may also affect activity of cells in the hypothalamic melanocortin system, which regulates caloric intake and metabolic rate in response to energy availability (Fan et al, 2005; Ramos et al, 2005). For example, activation of the dopamine D2 receptor increases expression of anorexic pro-opiomelanocortin mRNA and decreases expression of orexigenic neuropeptide Y mRNA within the arcuate nucleus of the hypothalamus (Pelletier and Simard, 1991; Tong and Pelletier, 1992). By increasing extracellular DA, DA reuptake inhibitors could indirectly increase D2 receptor activation and affect activity of neurons in the melanocortin system.
Part of the efficacy of drugs like BUP is that they affect multiple systems, sometimes resulting in favorable drug interactions that could not be predicted based on the individual effects of selective DA and NE reuptake inhibitors (Kaplan, 2005). An example of a drug interaction that was not predicted is the effect of subchronic DA+NE reuptake coadministration on IBAT temperature. During the light phase, subchronic DA+NE reuptake inhibition caused an average 0.4°C increase in IBAT temperature, even though temperature was essentially unaffected by DA reuptake inhibition and decreased by NE reuptake inhibition. Although adult humans do not have defined peripheral BAT deposits, recent evidence shows that brown adipocytes may be dispersed within white adipose tissue deposits. Additional studies linking human obesity to β3-adrenoceptor and UCP-1 genetic polymorphisms suggest a role for BAT in energy expenditure in humans (Avram et al, 2005).
This study emphasizes that although acute studies may infer long-term drug effects, they do not necessarily predict a drug's chronic effects. Even though the chronic studies presented here were limited (by method of administration and drug solubility) to 1 week of treatment, they were sufficient to illustrate differences between the acute and subchronic effects of catecholamine reuptake inhibitors. Further studies assessing the effects of chronic (2–3 week) drug treatment could potentially offer a more comprehensive analysis of the chronic effects of catecholamine reuptake inhibitors on energy balance, but may be confounded by different methods of drug administration. Even daily peripheral BUP injection may not produce the same neuronal adaptations as chronic BUP infusion via minipump, which produces more stable drug and metabolite levels (Ferris and Beaman, 1983; Klimek et al, 1985; Ascher et al, 1995). Increasing evidence suggests that the cellular and behavioral effects of chronically administered catecholamine reuptake inhibitors and psychostimulants also depend on whether chronically administered drugs are delivered via daily bolus injection or continuous infusion (Ansah et al, 1996; Davidson et al, 2005). Because previous studies on the cellular effects of chronic BUP were conducted in rodents that received BUP orally or via daily injection, further studies examining how chronic infusion of BUP or selective DA+NE reuptake inhibitors via osmotic minipump affects neuronal signaling are necessary to begin to elucidate the most probable mechanism through which catecholamine reuptake inhibition affects energy balance.
It has been established in vitro and in vivo that BUP inhibits both the DA and NE transporters (Nomikos et al, 1989; Ferris and Cooper, 1993; Wellman, 2005). We previously demonstrated that coadministration of selective DA+NE reuptake inhibitors produces similar effects on food intake and body weight as BUP (Billes and Cowley, 2007). We extend our previous finding by demonstrating that coadministration of DA+NE reuptake inhibitors mimics the effects of BUP on locomotor activity and interscapular temperature. This study also corroborates the modest effect of BUP on body weight in humans. More importantly, we illustrate the individual effects of selective DA or selective NE reuptake inhibition on activity and interscapular temperature and demonstrate that coadministration of selective DA+NE reuptake inhibitors increased activity and temperature and caused weight loss in lean mice. It remains to be determined whether catecholamine reuptake inhibition would have a greater effect on energy balance in an obese rodent model, as has been suggested by acute studies with BUP, NIS, and GBR (Billes and Cowley, 2007) and also sibutramine (Strack et al, 2002). The results of this study shed light on the mechanism through which catecholamine reuptake inhibitors cause weight loss and provide additional insight into the role of catecholamines in regulation of energy balance. The relevance for humans is not that combined catecholamine reuptake inhibitors may actually increase energy expenditure, but rather that they may prevent diet-induced decreases in adaptive thermogenesis, thereby facilitating weight loss.
References
Ansah TA, Wade LH, Shockley DC (1996). Changes in locomotor activity, core temperature, and heart rate in response to repeated cocaine administration. Physiol Behav 60: 1261–1267.
Appolinario JC, Bueno JR, Coutinho W (2004). Psychotropic drugs in the treatment of obesity: what promise? CNS Drugs 18: 629–651.
Ascher JA, Cole JO, Colin JN, Feighner JP, Ferris RM, Fibiger HC et al (1995). Bupropion: a review of its mechanism of antidepressant activity. J Clin Psychiatry 56: 395–401.
Avram AS, Avram MM, James WD (2005). Subcutaneous fat in normal and diseased states: 2. Anatomy and physiology of white and brown adipose tissue. J Am Acad Dermatol 53: 671–683.
Billes SK, Cowley MA (2007). Inhibition of dopamine and norepinephrine reuptake produces additive effects on energy balance in lean and obese mice. Neuropsychopharmacology 32: 822–834.
Butler AA, Marks DL, Fan W, Kuhn CM, Bartolome M, Cone RD (2001). Melanocortin-4 receptor is required for acute homeostatic responses to increased dietary fat. Nat Neurosci 4: 605–611.
Connoley IP, Liu YL, Frost I, Reckless IP, Heal DJ, Stock MJ (1999). Thermogenic effects of sibutramine and its metabolites. Br J Pharmacol 126: 1487–1495.
Cooper BR, Hester TJ, Maxwell RA (1980). Behavioral and biochemical effects of the antidepressant bupropion (Wellbutrin): evidence for selective blockade of dopamine uptake in vivo. J Pharmacol Exp Ther 215: 127–134.
Crowley VE, Yeo GS, O'Rahilly S (2002). Obesity therapy: altering the energy intake-and-expenditure balance sheet. Nat Rev Drug Discov 1: 276–286.
Davidson C, Lee TH, Ellinwood EH (2005). Acute and chronic continuous methamphetamine have different long-term behavioral and neurochemical consequences. Neurochem Int 46: 189–203.
Dong J, Blier P (2001). Modification of norepinephrine and serotonin, but not dopamine, neuron firing by sustained bupropion treatment. Psychopharmacology (Berl) 155: 52–57.
Fan W, Voss-Andreae A, Cao WH, Morrison SF (2005). Regulation of thermogenesis by the central melanocortin system. Peptides 26: 1800–1813.
Ferris RM, Beaman OJ (1983). Bupropion: a new antidepressant drug, the mechanism of action of which is not associated with down-regulation of postsynaptic beta-adrenergic, serotonergic (5-HT2), alpha 2-adrenergic, imipramine and dopaminergic receptors in brain. Neuropharmacology 22: 1257–1267.
Ferris RM, Cooper BR (1993). Mechanism of antidepressant activity of bupropion. J Clin Psychiatry Monograph 11: 2–14.
Foley KF, DeSanty KP, Kast RE (2006). Bupropion: pharmacology and therapeutic applications. Expert Rev Neurother 6: 1249–1265.
Frazer A, Benmansour S (2002). Delayed pharmacological effects of antidepressants. Mol Psychiatry 7 (Suppl 1): S23–S28.
Gadde KM, Xiong GL (2007). Bupropion for weight reduction. Expert Rev Neurother 7: 17–24.
Golozoubova V, Strauss F, Malmlof K (2006). Locomotion is the major determinant of sibutramine-induced increase in energy expenditure. Pharmacol Biochem Behav 83: 517–527.
Griffith JD, Carranza J, Griffith C, Miller LL (1983). Bupropion: clinical assay for amphetamine-like abuse potential. J Clin Psychiatry 44: 206–208.
Harkin A, O'Donnell JM, Kelly JP (2002). A study of VitalView for behavioural and physiological monitoring in laboratory rats. Physiol Behav 77: 65–77.
Harto-Truax N, Stern WC, Miller LL, Sato TL, Cato AE (1983). Effects of bupropion on body weight. J Clin Psychiatry 44: 183–186.
Hasegawa H, Meeusen R, Sarre S, Diltoer M, Piacentini MF, Michotte Y (2005). Acute dopamine/norepinephrine reuptake inhibition increases brain and core temperature in rats. J Appl Physiol 99: 1397–1401.
Hoffman BJ, Hansson SR, Mezey E, Palkovits M (1998). Localization and dynamic regulation of biogenic amine transporters in the mammalian central nervous system. Front Neuroendocrinol 19: 187–231.
Ioannides-Demos LL, Proietto J, McNeil JJ (2005). Pharmacotherapy for obesity. Drugs 65: 1391–1418.
Iversen LL (1971). Role of transmitter uptake mechanisms in synaptic neurotransmission. Br J Pharmacol 41: 571–591.
Izenwasser S, French D, Carroll FI, Kunko PM (1999). Continuous infusion of selective dopamine uptake inhibitors or cocaine produces time-dependent changes in rat locomotor activity. Behav Brain Res 99: 201–208.
Kaplan LM (2005). Pharmacological therapies for obesity. Gastroenterol Clin North Am 34: 91–104.
Klimek V, Nowak G, Czyrak A (1985). Central effects of repeated treatment with bupropion. Pol J Pharmacol Pharm 37: 243–252.
Liu YL, Connoley IP, Harrison J, Heal DJ, Stock MJ (2002). Comparison of the thermogenic and hypophagic effects of sibutramine's metabolite 2 and other monoamine reuptake inhibitors. Eur J Pharmacol 452: 49–56.
Liu YL, Connoley IP, Heal DJ, Stock MJ (2004). Pharmacological characterisation of the thermogenic effect of bupropion. Eur J Pharmacol 498: 219–225.
Lomax P, Daniel KA (1990). Cocaine and body temperature in the rat: effects of ambient temperature. Pharmacology 40: 103–109.
Lowell BB, Spiegelman BM (2000). Towards a molecular understanding of adaptive thermogenesis. Nature 404: 652–660.
Major GC, Doucet E, Trayhurn P, Astrup A, Tremblay A (2007). Clinical significance of adaptive thermogenesis. Int J Obes (London) 31: 204–212.
Missale C, Nash SR, Robinson SW, Jaber M, Caron MG (1998). Dopamine receptors: from structure to function. Physiol Rev 78: 189–225.
Mitchell HA, Ahern TH, Liles LC, Javors MA, Weinshenker D (2006). The effects of norepinephrine transporter inactivation on locomotor activity in mice. Biol Psychiatry 60: 1046–1052.
Nielsen JA, Shannon NJ, Bero L, Moore KE (1986). Effects of acute and chronic bupropion on locomotor activity and dopaminergic neurons. Pharmacol Biochem Behav 24: 795–799.
Nomikos GG, Damsma G, Wenkstern D, Fibiger HC (1989). Acute effects of bupropion on extracellular dopamine concentrations in rat striatum and nucleus accumbens studied by in vivo microdialysis. Neuropsychopharmacology 2: 273–279.
Pelletier G, Simard J (1991). Dopaminergic regulation of pre-proNPY mRNA levels in the rat arcuate nucleus. Neurosci Lett 127: 96–98.
Ramos EJ, Meguid MM, Campos AC, Coelho JC (2005). Neuropeptide Y, alpha-melanocyte-stimulating hormone, and monoamines in food intake regulation. Nutrition 21: 269–279.
Redolat R, Vidal J, Gomez MC, Carrasco MC (2005). Effects of acute bupropion administration on locomotor activity in adolescent and adult mice. Behav Pharmacol 16: 59–62.
Soroko FE, Mehta NB, Maxwell RA, Ferris RM, Schroeder DH (1977). Bupropion hydrochloride ((+/−) alpha-t-butylamino-3-chloropropiophenone HCl): a novel antidepressant agent. J Pharm Pharmacol 29: 767–770.
Spiegelman BM, Flier JS (2001). Obesity and the regulation of energy balance. Cell 104: 531–543.
Stahl SM, Pradko JF, Haight BR, Modell JG, Rockett CB, Learned-Coughlin S (2004). A review of the neuropharmacology of bupropion, a dual norepinephrine and dopamine reuptake inhibitor. Prim Care Companion J Clin Psychiatry 6: 159–166.
Strack AM, Shu J, Camacho R, Gorski JN, Murphy B, MacIntyre DE et al (2002). Regulation of body weight and carcass composition by sibutramine in rats. Obes Res 10: 173–181.
Tong Y, Pelletier G (1992). Role of dopamine in the regulation of proopiomelanocortin (POMC) mRNA levels in the arcuate nucleus and pituitary gland of the female rat as studied by in situ hybridization. Brain Res Mol Brain Res 15: 27–32.
Vassout A, Bruinink A, Krauss J, Waldmeier P, Bischoff S (1993). Regulation of dopamine receptors by bupropion: comparison with antidepressants and CNS stimulants. J Recept Res 13: 341–354.
Watson P, Hasegawa H, Roelands B, Piacentini MF, Looverie R, Meeusen R (2005). Acute dopamine/noradrenaline reuptake inhibition enhances human exercise performance in warm, but not temperate conditions. J Physiol 565: 873–883.
Wellman PJ (2005). Modulation of eating by central catecholamine systems. Curr Drug Targets 6: 191–199.
Zarrindast MR, Abolfathi-Araghi F (1992). Effects of bupropion on core body temperature of mice. Psychopharmacology (Berl) 106: 248–252.
Zarrindast MR, Hosseini-Nia T (1988). Anorectic and behavioural effects of bupropion. Gen Pharmacol 19: 201–204.
Acknowledgements
We thank Dr Kevin L Grove and Dr Gregory P Mark for helpful discussion and advice on this manuscript, as well as Joseph A Rathner, Puspha Sinnayah and Pablo Enriori for help with biotelemetry, and Nicholas Wallingford for technical assistance. This work was supported by grants from the National Institutes of Health (RR0163 and DK62202).
Author information
Authors and Affiliations
Corresponding author
Additional information
Disclosure/Conflicts of Interest
Michael A Cowley is chief scientific officer of, and owns stock in, Orexigen Therapeutics Inc. A company that is developing pharmaceutical approaches to treat obesity, and is developing combination therapies that include a formulation of bupropion. The work described in this manuscript was not supported by Orexigen Therapeutics Inc.
OHSU and Cowley have a significant financial interest in Orexigen Therapeutics Inc., a company that may have a commercial interest in the results of this research and technology. This potential conflict has been reviewed and managed by the OHSU Conflict of Interest in Research Committee and the Integrity Program Oversight Council.
Sonja K Billes has no conflicts of interest.
Rights and permissions
About this article
Cite this article
Billes, S., Cowley, M. Catecholamine Reuptake Inhibition Causes Weight Loss by Increasing Locomotor Activity and Thermogenesis. Neuropsychopharmacol 33, 1287–1297 (2008). https://doi.org/10.1038/sj.npp.1301526
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.npp.1301526