Abstract
Although nicotine is the main addictive chemical in tobacco, there have been few studies of pure nicotine self-administration in humans. The goal of this study was to test the parameters of an intravenous (IV) nicotine self-administration model using nicotine doses presumed to be within the range of those of average intake from cigarette smoking. Six male and four female smokers participated in a double-blind, placebo-controlled, crossover study, which consisted of one adaptation and three experimental sessions. In each experimental session, subjects were randomly assigned to one of the three doses of nicotine (0.1, 0.4, or 0.7 mg). The lowest nicotine dose, 0.1 mg, was chosen to be approximately half the amount of nicotine inhaled from one puff of a cigarette. During each experimental session, subjects first sampled the assigned nicotine dose and placebo and then had the opportunity to choose between nicotine and placebo for a total of six choices over a 90-min period. Out of six options, the average (SEM) number of nicotine choices were 3.0 (0.48) for 0.1 mg, 4.7 (0.48) for 0.4 mg and 4.5 (0.46) for 0.7 mg, indicating a significant effect of nicotine dose on nicotine choice. Both the 0.4 and 0.7, but not the 0.1 mg, nicotine doses were preferred to placebo. These higher doses also produced increases in heart rate, blood pressure, and ratings of drug liking and high. Overall, these findings indicate that smokers chose both the 0.4 and the 0.7 mg nicotine doses over placebo. Our model may be useful in the evaluation of the effects of both behavioral and pharmacological manipulations on nicotine self-administration in humans.
Similar content being viewed by others
INTRODUCTION
There have been few studies of pure nicotine self-administration in humans despite the fact that nicotine is the main addictive chemical in tobacco (Rose and Corrigall, 1997; Benowitz, 1999; Rose et al, 2001; Le Foll and Goldberg 2006). Two paradigms have used nicotine replacement therapy (NRT) products, nicotine nasal spray, and nicotine gum, to assess preference for nicotine over placebo in abstinent smokers evaluated in human laboratory settings (Perkins et al, 1996; Hughes et al, 2000). These experiments have not shown consistent preferences for nicotine over placebo. In contrast, users of other drugs of abuse, including cocaine, amphetamines, benzodiazepines, and alcohol, consistently prefer active drug over placebo in the human laboratory context (de Wit and McCracken, 1990; Foltin and Fischman, 1992; Troisi II et al, 1993; Hatsukami et al, 1994; Tancer and Johanson, 2003; Stoops et al, 2005). Two important factors could account for the lack of nicotine preference in the well-controlled studies of Perkins et al (1996) and Hughes et al (2000). First, the two routes of nicotine used could lead to aversive effects including local irritation and nasal burning (nasal spray) or taste, local irritation, and hiccups (nicotine gum). In recent studies of preferences for NRTs, substantial proportions of smokers do not prefer nicotine gum or nasal spray (West et al, 2001; Schneider et al, 2004, 2005). Second, the slower nicotine delivery via nicotine gum, compared to cigarette smoking, may diminish reinforcing effects (de Wit et al, 1992; Nelson et al, 2006).Thus, other routes, such as the intravenous (IV) one, may be more optimum for nicotine self-administration studies.
A series of studies have tested IV nicotine self-administration in smokers (Henningfield and Goldberg, 1983; Henningfield et al, 1983; Harvey et al, 2004). In the Harvey et al (2004) study, during 3 h sessions, IV nicotine (0.75, 1.5, and 3.0 mg/injection) and saline were available concurrently for abstinent male cigarette smokers who, on average, smoked 29 cigarettes/day. To receive the injections, smokers had to pull a lever according to a fixed-ratio requirement ranging from 10 to 1600 (Harvey et al, 2004). Smokers preferred nicotine injections over saline administration for all three nicotine doses. Moreover, when the work requirement was higher, fixed-ratio values equal or over 200 lever pulls, rates of responding were significantly greater for nicotine than for saline. Importantly, the nicotine doses used in the Harvey et al study delivered higher than the usual nicotine intake of an average smoker, which is, on average, 1–2 cigarettes/h or 1–4 mg nicotine/h (Benowitz and Jacob, 1990). Nicotine dose may be a critical factor since in a previous study, IV nicotine doses over 1.5 mg were rated similar to cocaine or amphetamines by smokers who have used stimulants (Chausmer et al, 2003). Thus, whether smokers prefer IV nicotine over placebo in nicotine doses within the range of those of average intake from smoking remains to be determined. To address this question, this study used a choice procedure in which smokers were able to choose between various IV nicotine doses or saline. The nicotine doses chosen were 0.1, 0.4, and 0.7 mg, relative to placebo (saline). The 0.1 mg dose is less than the amount of nicotine inhaled from one puff of a cigarette (Djordjevic et al, 2000). The 0.7 mg is close to the minimum nicotine dose that has been shown to be self-administered in the Harvey et al (2004). Thus, this study extended earlier IV nicotine self-administration studies by testing lower doses of nicotine presumed to be within the range of nicotine doses delivered with cigarette puffs and including both male and female smokers, who were less heavy cigarette smokers and without a history of any drug and alcohol dependence.
METHODS
Subjects
Six male and four female non-treatment seeking smokers were recruited from the New Haven area (three African-Americans, six Caucasians, one Hispanic). Four additional participants were enrolled but dropped out of the study before participating in experimental sessions and were not included in the analyses. The reasons for dropping out were high baseline blood pressure (n=2), fear of the IV catheter (n=1) and positive urine toxicology for drugs of abuse (n=1). The average age (SD) of the participants was 35.0 (10.1). On average, participants smoked 17.4 (4.3) cigarettes/day, and had a Fagerstrom test for Nicotine Dependence (Heatherton et al, 1991) score of 6.7 (1.5). Participants had normal physical, laboratory and psychiatric examinations. None of the participants ever met criteria for drug abuse or dependence for any substances other than nicotine, as established by psychiatric examination (APA, 1994). Subjects had urine drug screening before each session to rule out recent drug use. All participants signed informed consent forms before their entry into the study. All the sessions were conducted in the Biostudies Unit located at the VA Connecticut Healthcare System (West Haven campus) and participants were paid for participation. This study was approved by the VA Connecticut Healthcare System Human Subjects Subcommittee.
Procedures
This outpatient, double-blind, crossover study had one adaptation and three experimental sessions. For all of these sessions, subjects were instructed not to smoke after midnight the night before sessions. Smoking abstinence was verified using breath carbon monoxide levels (<10 parts per million (ppm)) and plasma nicotine levels <5 ng/ml. Before the beginning of each session, an indwelling IV catheter was placed in the subject's antecubital vein for nicotine infusion, blood drawing and as a safety precaution. In the adaptation session, subjects first received an IV saline injection followed by three escalating doses of IV nicotine (0.1, 0.4, or 0.7 mg), given 30 min apart. Each injection was given over 30 s and ensured that subjects tolerated the IV saline and nicotine doses that were used in the experimental sessions. In each experimental session, subjects were randomly assigned to one of the three doses of nicotine (0.1, 0.4, or 0.7 mg). At the beginning of each experimental session, subjects first sampled the assigned nicotine dose and placebo (saline), randomly labeled A or B. The label assigned to nicotine was randomly determined for each session by the research pharmacist (SY), and the research team was blind to the assignment. Starting 15 min after the second sample dose, subjects had the opportunity to choose between drug A or B every 15 min, for a total of six opportunities over a 90-min period. Immediately after subjects made their choice, drugs were administered using an infusion pump over 30 s. Cardiac rhythm was monitored continuously during sessions, and 12-lead ECGs were obtained before and at the end of the session. The sessions started at 0800 hour and were 2–7 days apart to minimize any carryover effects from nicotine.
Drugs
Nicotine administration
Nicotine stock solution vials were prepared by dissolving nicotine bitartrate dihydrate powder in 0.9% sodium chloride and passed through 0.22 μm filters to target a 1 mg/ml concentration. The amount of nicotine bitartrate dehydrate powder was adjusted by molecular weight to reflect nicotine-free base. Each batch of nicotine solution was tested for pyrogenicity, sterility and analyzed by quantitative assay, which yielded satisfactory results in all cases. An investigational new drug application was obtained from the Food and Drug Administration for IV nicotine.
For adaptation sessions, four 10-ml syringes were prepared to familiarize subjects with ascending doses of nicotine injections. All syringes contained 5 ml of solution and appeared identical except for the labels were numbers as 1, 2, 3, and 4. Syringe 1 contained 5 ml of 0.9% NaCl and the subsequent syringes contained 0.1, 0.4, and 0.7 mg of nicotine with enough 0.9% NaCl added to make the final volume of 5 ml. Each injection was given over a 30-s period.
For experimental sessions, two syringes were prepared in randomized, double-blinded fashion. Two 60-ml syringes were marked as either A or B on identical-looking IV labels containing 46 ml of solution in each syringe. Depending on the randomization, one of the syringes contained an active nicotine dose of 0.1 mg/5 ml, 0.4 mg/5 ml, or 0.7 mg/5 ml. The total amount of nicotine solution in the active syringe accounted for the line flush and seven possible doses, one for the sample dose, and the six optional doses. Matching placebo syringes contained 46 ml of 0.9% NaCl. Syringes were capped, labeled and dispensed to the study staff by the research pharmacy.
Outcome Measures
The study included behavioral, biochemical, physiological, and subjective measures. The behavioral measure was the number of nicotine choices under the three nicotine doses (0.1, 0.4, or 0.7 mg). The biochemical measure was plasma nicotine concentrations to verify abstinence from smoking. For plasma nicotine levels, blood samples were obtained at the beginning of each session.
The physiological measures were systolic and diastolic blood pressure and heart rate. These measures were taken at the beginning and end of each session. Physiological measures were also taken just before injection and 1, 5, 10, and 15 min after each dose delivery. For the sample doses, additional measures were obtained at 2, 3, and 8 min after dose deliveries.
The three subjective measures were: the Drug Effects Questionnaire (DEQ), a visual analog scale (VAS) questionnaire for nicotine withdrawal, the Profile of Mood States (POMS), and the positive and negative affect schedule (PANAS). The DEQ, used to assess the acute subjective effects of nicotine, consists of six items: feeling drug effects, high, good effects, bad effects, head rush and like the drug. Participants rated these items on a 100 mm scale, from 0 ‘not at all’ to 100 ‘extremely.’ The DEQ was given 1, 5, and 10 min after each dose delivery. For the two sample doses, additional DEQ measures were obtained at 3 and 8 min after each dose. The DEQ was adapted from VAS questionnaires used in previous IV nicotine studies (Soria et al, 1996; Jones et al, 1999).
The VAS was used to assess nicotine withdrawal and included 8-items adapted from the nicotine withdrawal symptom checklist (Hughes and Hatsukami, 1986). The items were cigarette craving, irritability/anger, anxiety, difficulty concentrating, restlessness, increased appetite, depressed mood, and insomnia (Hughes and Hatsukami, 1986, 1997). Participants were asked to rate each symptom on a scale from 0 (not present) to 100 (severe).
The POMS is a 72-item rating scale used to measure the effects of medication treatments on mood (McNair et al, 1971). The POMS has six subscales: (1) composed-anxious; (2) agreeable-hostile; (3) elated-depressed; (4) confident-unsure; (5) energetic-tired; and (6) clear headed-confused. The VAS withdrawal scale and POMS were given two times at the beginning and end of each session. The PANAS is a 20-item scale, which assesses both negative and positive affective states (Watson et al, 1988). Participants rate adjectives describing affective states on a scale of 1–5 using a specified time period (eg now, today and past week). The PANAS has been shown to be sensitive to the affective symptoms of tobacco withdrawal (Kenford et al, 2002).
Data Analysis
All analyses were conducted using the Statistical Analysis System, version 9.13. All main effects and interactions were considered statistically significant at p<0.05. Repeatedly measured outcomes were evaluated using mixed effects ANOVA implemented through the MIXED procedure. For the number of nicotine deliveries, the model included fixed effects of treatment (0.1, 0.4, and 0.7 mg nicotine or saline), as well as sequence and treatment by sequence interactions to assess for carryover and order effects. Subject was treated as a random effect. For the POMS, PANAS, and the VAS data, the change in scores from baseline were used as the dependent measure in the model. For blood pressure, heart rate, and DEQ data, sample doses were analyzed with a similar model, which also included a fixed main effect for time of measurement from nicotine or saline injection, as well as treatment-by-time interactions. For the sample dose blood pressure, heart rate and DEQ measurements, significant main effects were followed up by post hoc comparisons of each treatment relative to placebo for different time points. To account for multiple testing, for these comparisons, statistical significance was set at p<0.01.
RESULTS
Nicotine Self-Administration
Nicotine vs Placebo
The relative preference for nicotine to placebo was equal for the 0.1 mg dose (mean difference=−0.20, SEM=1.2), while nicotine was significantly more preferred than placebo at the 0.4 mg dose, t(9)=4.67, p=0.0012 (mean difference=3.80, SEM=0.81), and the 0.7 mg dose, t(9)=3.75, p=0.0046 (mean difference=3.20, SEM=0.85).
Dose Response
The number of nicotine dose choices increased significantly with increasing nicotine dose, F(212.1)=4.98, p=0.0264. The average number of covariate-adjusted (treatment sequence) nicotine self-administrations were 3.0 (0.48) for 0.1 mg, 4.7 (0.48) for 0.4 mg, and 4.5 (0.46) for 0.7 mg. Pairwise comparisons showed that both 0.4 and 0.7 mg doses were chosen more often than the 0.1 mg dose (p<0.05).
Physiological Effects
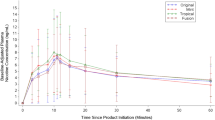
For sample dose deliveries (Figure 1), a significant treatment effect was observed for heart rate (F(3, 339)=5.2; p<0.01) and systolic blood pressure (F(3, 339)=4.6; p<0.01) measurements. Pairwise comparisons showed several differences (p<0.05) between doses at multiple time points (see Figure 1, differences are denoted by symbols). For both heart rate and systolic blood pressure, the 0.7 mg nicotine dose yielded higher levels, as compared to both the 0.1 mg nicotine or placebo doses. Further, the 0.4 mg nicotine dose produced greater increases on these same physiological indices compared to the 0.1 mg nicotine dose. For systolic blood pressure, the 0.4 mg nicotine dose had a higher level relative to placebo.
The average (±SEM) heart rate, systolic and diastolic pressure responses to a sample nicotine dose (0.1, 0.4, and 0.7 mg) and saline administration. Measurements were taken just before and 1, 3, 5, 8, and 10 min after dose delivery. Some of the error bars are not shown for clarity. Significant treatment differences from placebo at each time point (p<0.01) are indicated by an asterisks (*) for the 0.7 mg and plus (+) for the 0.4 mg nicotine dose.
Subjective Effects
DEQ
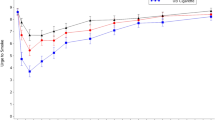
For sample dose deliveries (Figure 2), a significant treatment effect was observed for the rating of feel drug effects (F(3, 275)=4.8; p<0.01), high (F(3, 275)=2.8; p<0.05), head rush (F(3, 275)=4.6; p<0.01), bad effects (F(3, 275)=2.7; p<0.05), good effects (F(3, 275)=4.9; p<0.01), and like drug effects (F(3, 275)=3.9; p<0.01). Pairwise comparisons showed that for all items, the 0.7 mg nicotine dose yielded higher ratings than either the 0.4 mg nicotine, 0.1 mg nicotine, or placebo doses. For the rating of feel bad effects and drug effects 0.4 mg nicotine dose exceeded placebo; for feeling drug effects, good effects and high, the 0.4 mg nicotine dose exceeded the 0.1 mg nicotine.
The average scores (±SEM) on selected subjective measures in response to a sample nicotine dose (0.1, 0.4, and 0.7 mg) and saline administration. Measurements were taken at 1, 3, 5, 8, and 10 min after dose delivery. Some of the error bars are not shown for clarity. Significant treatment differences from placebo at each time point (p<0.01) are indicated by an asterisks (*) for the 0.7 mg nicotine dose.
POMS
Among the POMS subscales, the energetic-tired subscale showed a significant treatment effect (F(2, 16)=3.6; p=0.05). Pairwise comparisons revealed that the 0.4 mg nicotine dose had a superior level on the energetic-tired subscale relative to the 0.1 mg nicotine dose, indicating greater tiredness under 0.1 mg nicotine dose.
PANAS
Change in the negative affect subscale of the PANAS showed a significant treatment effect (F(2, 21)=3.9; p<0.05). Pairwise comparisons of the change scores showed that the 0.1 mg nicotine dose showed greater change than the 0.7 mg nicotine dose condition. Under the 0.1 mg nicotine condition, the average score increased 0.5 points, while under 0.4 and 0.7 mg nicotine conditions, the score decreased 0.2 and 1.3 points, respectively.
DISCUSSION
The goal of this study was to evaluate IV nicotine self-administration in doses that are presumed to be within the range of nicotine intake from cigarette puffs. The 0.4 mg nicotine dose, equivalent to a few puffs of a cigarette, was chosen over saline nearly 80% of the time (ie mean 4.7 out of 6 choices). Similarly, the 0.7 mg nicotine dose, approximately equivalent to nicotine delivered by smoking one-half of a cigarette, was chosen over saline 75% of the time (ie mean 4.5 out of 6 choices). Both 0.4 and 0.7 mg nicotine doses produced subjective effects including ‘good effects’ and ‘drug liking,’ consistent with the abuse liability of nicotine. While the 0.7 mg dose induced greater ‘drug liking’ and ‘good effects’ than the 0.4 mg nicotine dose, smokers self-administered both doses at a similar rate. This plateau in dose response for nicotine self-administration is consistent with preclinical studies (Rose and Corrigall, 1997), as well as human studies (Henningfield and Goldberg, 1983; Henningfield et al, 1983; Harvey et al, 2004). In the Harvey et al study, the number of nicotine administrations was highest under the 0.75 mg condition, relative to 1.5 and 2.0 mg. This plateau may be due to satiety or aversive effects associated with higher doses of nicotine (Harvey et al, 2004).
Our findings extend previous studies, which demonstrated that smokers chose 0.75, 1.5, and 3 mg nicotine deliveries over placebo under a progressive-ratio work schedule (Henningfield and Goldberg, 1983; Henningfield et al, 1983; Harvey et al, 2004). Since these nicotine doses were higher than the average nicotine intake by cigarette puffs, it was of interest to examine self-administration of smaller nicotine doses. In addition, the study samples in these studies included men some of whom had illicit drug use histories, including cocaine and amphetamines, and on average smoked 29 cigarettes/day. This study sample raised questions of whether self-administration of IV nicotine would be limited to those who were heavy smokers or illicit drug use histories. Our study addressed these concerns by including both male and female smokers who smoked fewer cigarettes, 17 cigarettes/day, and who had no history of illicit drug use. Further, we used nicotine doses that produced nicotine delivery comparable to those with regular smoking. Our findings demonstrate that IV nicotine is reinforcing in doses that are relevant to smoking in overnight abstinent smokers.
The 0.1 mg nicotine dose was not self-administered more than placebo, suggesting that the 0.1 mg nicotine was subthreshold for reinforcement. These findings are consistent with those reported for threshold for nicotine discrimination. In smokers and nonsmokers, the threshold for nicotine discrimination administered via nasal spray was approximately 0.2 mg (Perkins et al, 2001). Interestingly, the 0.2 mg nicotine is approximately equal to the amount of nicotine delivered by smoking a puff of a cigarette (Djordjevic et al, 2000). The 0.1 mg dose may be useful in evaluating manipulations that enhance nicotine's reinforcing effects.
As expected, IV nicotine produced dose-dependent subjective and physiological effects in response to sample doses. Since the dose deliveries following the sample doses were optional, our study could not accurately address the responses to repeated nicotine deliveries. However, the data with nearly 80% of the nicotine doses delivered suggest a modest increase in heart rate and blood pressure in response to repeated nicotine deliveries (data not presented). These findings support the safety of our self-administration model. In addition, 0.7 mg of nicotine, compared to 0.4 or 0.1 mg of nicotine, produced improvement in negative mood and decreased tiredness. These findings are consistent with the effects of nicotine from previous studies.
What are the implications of our findings? As mentioned before, for other drugs of abuse including cocaine, amphetamines, benzodiazepines, and alcohol, reliable human self-administration models have been developed. These models have utility in evaluating the effects of both behavioral and pharmacological manipulations on drug reinforcement. Our findings demonstrate that the model used in our study could serve to examine nicotine self-administration. Especially encouraging was that the study procedures including the IV nicotine doses were well tolerated by both male and female smokers. These findings support the feasibility of our model to examine nicotine self-administration in humans. Our IV nicotine self-administration model may have utility in developing and testing new behavioral and pharmacological treatments for nicotine addiction. In addition, our model may also be useful in neuroimaging studies, where IV route is preferred over other routes of nicotine administration.
References
American Psychiatric Association (1994). Diagnostic and Statistical Manual of Mental Disorders: DSM-IV, 4th edn. American Psychiatric Association: Washington, DC.
Benowitz NL (1999). Nicotine addiction. Prim Care 26: 611–631.
Benowitz NL, Jacob Pd (1990). Intravenous nicotine replacement suppresses nicotine intake from cigarette smoking. J Pharmacol Exp Therap 254: 1000–1005.
Chausmer AL, Smith BJ, Kelly RY, Griffiths RR (2003). Cocaine-like subjective effects of nicotine are not blocked by the D1 selective antagonist ecopipam (SCH 39166). Behav Pharmacol 14: 111–120.
de Wit H, Bodker B, Ambre J (1992). Rate of increase of plasma drug level influences subjective response in humans. Psychopharmacology (Berlin) 107: 352–358.
de Wit H, McCracken SG (1990). Ethanol self-administration in males with and without an alcoholic first-degree relative. Alcohol Clin Exp Res 14: 63–70.
Djordjevic MV, Stellman SD, Zang E (2000). Doses of nicotine and lung carcinogens delivered to cigarette smokers. J Natl Cancer Inst 92: 106–111.
Foltin RW, Fischman MW (1992). Self-administration of cocaine by humans: choice between smoked and intravenous cocaine. J Pharmacol Exp Ther 261: 841–849.
Harvey DM, Yasar S, Heishman SJ, Panlilio LV, Henningfield JE, Goldberg SR (2004). Nicotine serves as an effective reinforcer of intravenous drug-taking behavior in human cigarette smokers. Psychopharmacology (Berlin) 175: 134–142.
Hatsukami DK, Thompson TN, Pentel PR, Flygare BK, Caroll ME (1994). Self-administration of smoked cocaine. Exp Clin Psychopharmacol 2: 115–125.
Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom KO (1991). The Fagerstrom Test for Nicotine Dependence: a revision of the Fagerstrom Tolerance Questionnaire. Br J Addictions 86: 1119–1127.
Henningfield JE, Goldberg SR (1983). Control of behavior by intravenous nicotine injections in human subjects. Pharmacol Biochem Behav 19: 1021–1026.
Henningfield JE, Miyasato K, Jasinski DR (1983). Cigarette smokers self-administer intravenous nicotine. Pharmacol Biochem Behav 19: 887–890.
Hughes JR, Hatsukami D (1986). Signs and symptoms of tobacco withdrawal. Arch Gen Psychiatry 43: 289–294.
Hughes JR, Hatsukami DK (1997). Effects of three doses of transdermal nicotine on post-cessation eating, hunger and weight. J Subst Abuse 9: 151–159.
Hughes JR, Rose GL, Callas PW (2000). Do former smokers respond to nicotine differently from never smokers? A pilot study. Nicotine Tob Res 2: 255–262.
Jones HE, Garrett BE, Griffiths RR (1999). Subjective and physiological effects of intravenous nicotine and cocaine in cigarette smoking cocaine abusers. J Pharmacol Exp Ther 288: 188–197.
Kenford SL, Smith SS, Wetter DW, Jorenby DE, Fiore MC, Baker TB (2002). Predicting relapse back to smoking: contrasting affective and physical models of dependence. J Consult Clin Psychol 70: 216–227.
Le Foll B, Goldberg SR (2006). Nicotine as a typical drug of abuse in experimental animals and humans. Psychopharmacology (Berlin) 184: 367–381.
McNair D, Lorr M, Dropperman L (1971). EITS Manual for the Profile of Mood States. Educational and Industrial Testing Services: San Diego, CA.
Nelson RA, Boyd SJ, Ziegelstein RC, Herning R, Cadet JL, Henningfield JE et al (2006). Effect of rate of administration on subjective and physiological effects of intravenous cocaine in humans. Drug Alcohol Depend 82: 19–24.
Perkins KA, Fonte C, Sanders M, Meeker J, Wilson A (2001). Threshold doses for nicotine discrimination in smokers and non-smokers. Psychopharmacology (Berlin) 155: 163–170.
Perkins KA, Grobe JE, Weiss D, Fonte C, Caggiula A (1996). Nicotine preference in smokers as a function of smoking abstinence. Pharmacol Biochem Behav 55: 257–263.
Rose JE, Behm FM, Westman EC (2001). Acute effects of nicotine and mecamylamine on tobacco withdrawal symptoms, cigarette reward and ad lib smoking. Pharmacol Biochem Behav 68: 187–197.
Rose JE, Corrigall WA (1997). Nicotine self-administration in animals and humans: similarities and differences. Psychopharmacology 130: 28–40.
Schneider NG, Olmstead RE, Nides M, Mody FV, Otte-Colquette P, Doan K et al (2004). Comparative testing of 5 nicotine systems: initial use and preferences. Am J Health Behav 28: 72–86.
Schneider NG, Terrace S, Koury MA, Patel S, Vaghaiwalla B, Pendergrass R et al (2005). Comparison of three nicotine treatments: initial reactions and preferences with guided use. Psychopharmacology (Berlin) 182: 545–550.
Soria R, Stapleton JM, Gilson SF, Sampson-Cone A, Henningfield JE, London ED (1996). Subjective and cardiovascular effects of intravenous nicotine in smokers and non-smokers. Psychopharmacology 128: 221–226.
Stoops WW, Lile JA, Fillmore MT, Glaser PE, Rush CR (2005). Reinforcing effects of methylphenidate: influence of dose and behavioral demands following drug administration. Psychopharmacology (Berlin) 177: 349–355.
Tancer M, Johanson CE (2003). Reinforcing, subjective, and physiological effects of MDMA in humans: a comparison with d-amphetamine and mCPP. Drug Alcohol Depend 72: 33–44.
Troisi II JR, Critchfield TS, Griffiths RR (1993). Buspirone and lorazepam abuse liability in humans: behavioral effects, subjective effects and choice. Behav Pharmacol 4: 217–230.
Watson D, Clark LA, Tellegen A (1988). Development and validation of brief measures of positive and negative affect: the PANAS scales. J Pers Soc Psychol 54: 1063–1070.
West R, Hajek P, Nilsson F, Foulds J, May S, Meadows A (2001). Individual differences in preferences for and responses to four nicotine replacement products. Psychopharmacology (Berlin) 153: 225–230.
Acknowledgements
This research was supported by the Veterans Administration Mental Illness Research, Education and Clinical Center (MIRECC) and the National Institute on Drug Abuse (NIDA) grants R01-DA 14537, K01-DA 19446 (MM). We thank Ellen Mitchell, RN and Stacy Minnix for excellent technical assistance.
Author information
Authors and Affiliations
Corresponding author
Additional information
DISCLOSURE
The authors declare that, except for income received from my primary employer, no financial support or compensation has been received from any individual or corporate entity over the past 3 years for research or professional service and there are no personal financial holdings that could be perceived as constituting a potential conflict of interest.
Rights and permissions
About this article
Cite this article
Sofuoglu, M., Yoo, S., Hill, K. et al. Self-Administration of Intravenous Nicotine in Male and Female Cigarette Smokers. Neuropsychopharmacol 33, 715–720 (2008). https://doi.org/10.1038/sj.npp.1301460
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.npp.1301460
Keywords
This article is cited by
-
Development of pulsed intravenous nicotine infusions as a model for inhaled nicotine in humans
Psychopharmacology (2022)
-
Threshold dose for intravenous nicotine self-administration in young adult non-dependent smokers
Psychopharmacology (2021)
-
Intravenous Nicotine Self-Administration in Smokers: Dose–Response Function and Sex Differences
Neuropsychopharmacology (2016)
-
Reinforcing effectiveness of nicotine in nonhuman primates: effects of nicotine dose and history of nicotine self-administration
Psychopharmacology (2016)
-
Threshold dose for discrimination of nicotine via cigarette smoking
Psychopharmacology (2016)