Abstract
Elevated signs of anxiety are observed in both humans and rodents during withdrawal from chronic as well as acute ethanol exposure, and it represents an important motivational factor for ethanol relapse. Several reports have suggested the involvement of brain adenosine receptors in different actions produced by ethanol such as motor incoordination and hypnotic effects. In addition, we have recently demonstrated that adenosine A1 receptors modulate the anxiolytic-like effect induced by ethanol in mice. In the present study, we evaluated the potential of adenosine A1 and A2A receptor agonists in reducing the anxiety-like behavior during acute ethanol withdrawal (hangover) in mice. Animals received a single intraperitoneal administration of saline or ethanol (4 g/kg) and were tested in the elevated plus maze after an interval of 0.5–24 h. The results indicated that hangover-induced anxiety was most pronounced between 12 and 18 h after ethanol administration, as indicated by a significant reduction in the exploration of the open arms of the maze. At this time interval, ethanol was completely cleared. The acute administration of ‘nonanxiolytic’ doses of adenosine and the selective adenosine A1 receptor agonist 2-chloro-N6-cyclopentyladenosine (CCPA), but not the adenosine A2A receptor agonist N6-[2-(3,5-dimethoxyphenyl)-2-(2-methylphenyl)ethyl]adenosine (DPMA), at the onset of peak withdrawal (18 h), reduced this anxiogenic-like response. In addition, the effect of CCPA on the anxiety-like behavior of ethanol hangover was reversed by pretreatment with the selective adenosine A1 receptor antagonist 8-cyclopentyl-1,3-dipropylxanthine (DPCPX). These results reinforce the notion of the involvement of adenosine receptors in the anxiety-like responses and indicate the potential of adenosine A1 receptor agonists to reduce the anxiogenic effects during ethanol withdrawal.
Similar content being viewed by others
INTRODUCTION
Withdrawal is a defining characteristic of drug dependence and is often characterized by impaired physiological function and enhanced negative affect, symptoms strongly associated with relapse (Cloninger, 1987). The symptomatic profile of ethanol withdrawal has been well established in both humans and other animal subjects. Signs of withdrawal from ethanol, such as nausea, tremors, hyperthermia, tachycardia, irritability, anxiety, and insomnia, are most severe early in the withdrawal period following extended periods of alcohol consumption (for a review, see Finn and Crabbe, 1997). A milder form of ethanol withdrawal, called acute ethanol withdrawal (or hangover), can occur following a single ingestion of substantial amounts of alcohol. Physiological symptoms of hangover in humans include headache, nausea, diarrhea, anorexia, fatigue, and tremor (for a reviews, see Smith and Barnes, 1983; Wiese et al, 2000), along with psychological signs that include anxiety, guilt, and depression (Smith and Barnes, 1983; Bogin et al, 1986).
Models of ethanol withdrawal in adult rodents have consistently demonstrated increases in anxiety-like behavior during the withdrawal period after chronic exposure to ethanol (File et al, 1991, 1993; Lal et al, 1991; Knapp et al, 1993; Gatch et al, 1999; Gatch and Lal, 2001). In contrast, few studies have examined the presence of withdrawal symptoms following a single dose of ethanol in rodents. Gauvin et al (1992, 1993), using the anxiogenic drug pentylenetetrazol (PTZ) in the drug-discrimination task, have shown that rats injected intraperitoneally (i.p.) with high doses of ethanol (3 and 4 g/kg) demonstrate high levels of PTZ-induced lever-pressing responses when tested 9–18 h after acute ethanol challenge, suggesting that this response reflects hangover-related anxiety. Recently, some reports have demonstrated that, 18 h after the acute administration of ethanol (4 g/kg, i.p.), adult male rats present a reduced exploration of the open arms in the elevated plus maze (Doremus et al, 2003), and significant social suppression in the social interaction test (Varlinskaya and Spear, 2004), a response pattern that is consistent with an anxiogenic profile (File et al, 1976; Pellow et al, 1985). Moreover, Morse et al (2000) have demonstrated that the hangover following an acute injection of high doses of ethanol (3–4 g/kg) produces a significant conditioned place aversion in the rat. Contrasting with these results, Gauvin et al (1997) have demonstrated that although acute administration of high ethanol doses, most typically, induces conditioned place aversions, the repeated pairing of ethanol hangover with the initially nonpreferred side of the chambers results in the development of a conditioned place preference in rats, suggesting that, in some cases, the hangover procedure did not induce aversive response. However, to our knowledge, there are no studies reporting the anxiety-related symptoms of hangover or any measures to counteract this response in mice.
An increasing amount of evidence suggests that adenosine receptors mediate important actions of ethanol in both humans and rodents. Adenosine functions as a neuromodulator in the central nervous system (CNS), acting through cell-surface receptors (Cunha, 2001). At the moment, four adenosine receptor subtypes (A1, A2A, A2B, and A3) have been cloned and characterized from several mammalian species, including humans and mice, and they all belong to the G-protein-coupled receptor (GPCR) family (Fredholm et al, 2001). Acute exposure to ethanol increases the concentration of extracellular adenosine (Nagy et al, 1990; Krauss et al, 1993). Moreover, there is considerable evidence that the co-administration of caffeine (a nonselective adenosine receptor antagonist) can reduce sleep and psychomotor performance impairment associated with moderate-to-high ethanol doses in humans (Franks et al, 1975; Fillmore and Vogel-Sprott, 1995; Liguori and Robinson, 2001; Drake et al, 2003). Furthermore, the combined administration of caffeine and alcohol can increase the development of alcohol tolerance (Fillmore, 2003). In rodents, many studies using selective adenosine receptor agonists and antagonists have consistently demonstrated that adenosine receptors, localized in brain areas essential for motor control such as striatum, the cerebellum, and the motor cortex, are the primary site where adenosine modulates the incoordination induced by ethanol (Meng and Dar, 1995; Barwick and Dar, 1998; Dar, 2001). Additionally, we have recently demonstrated that the blockade of adenosine receptors inhibits the development of rapid tolerance to ethanol-induced motor impairment in mice (Batista et al, 2005).
Other studies suggest that adenosine receptors can modulate some of the signs of ethanol withdrawal, such as tremors and seizures. Concas et al (1994) have described a significant reduction of ethanol withdrawal syndrome in rats after treatment with the selective adenosine A1 receptor agonist 2-chloro-N6-cyclopentyladenosine (CCPA). Kaplan et al (1999) have demonstrated similar results for the adenosine A1 receptor agonist R-N6(phenylisopropyl)adenosine (R-PIA) and the adenosine A2A receptor agonist 2-p-(2-carboxethyl)phenylethyl-amino-5′-N-ethylcarboxamidoadenosine (CGS 21680) in attenuating the ethanol withdrawal in mice.
On the other hand, many studies have pointed to a direct involvement of adenosine in anxiety (for a review, see Millan, 2003). Mice lacking adenosine A1 receptors display enhanced anxiety (Johansson et al, 2001; Giménez-Llort et al, 2002; Lang et al, 2003) and the anxiogenic actions of adenosine receptor antagonists, such as caffeine, in both rodents and humans, have generally been attributed to the blockade of adenosine A1 receptors (Pellow et al, 1985; Commissaris et al, 1990; Jain et al, 1995; Florio et al, 1998). Although no consistent evidence for the anxiolytic-like effects of adenosine A2A receptor stimulation in rodents has, to date, been obtained (Jain et al, 1995; El Yacoubi et al, 2000), some authors have demonstrated that mice lacking adenosine A2A receptors appeared to be more ‘anxious’ and ‘aggressive’ than A2AR+/+ mice (Ledent et al, 1997). Indeed, clinical studies have found a significant association between polymorphisms on the adenosine A2A receptor gene and panic disorder as well as the self-reported anxiety after acute caffeine administration (Alsene et al, 2003; Hamilton et al, 2004). In addition, we have recently demonstrated that the activation of adenosine A1 receptors, but not adenosine A2A receptors, mediates the anxiolytic-like effect induced by ethanol in the elevated plus maze in mice, since the prior administration of ‘nonanxiogenic’ doses of caffeine and the selective adenosine A1 receptor antagonist 8-cyclopentyl-1,3-dipropylxanthine (DPCPX) significantly reduced the anxiolytic-like effect of ethanol (1.2 g/kg, i.p.). Moreover, anxiolytic-like response was observed by the co-administration of ‘nonanxiolytic’ doses of CCPA (0.125 mg/kg, i.p.) and ethanol (0.6 g/kg, i.p.) (Prediger et al, 2004).
We therefore investigated the occurrence of anxiety-like behavior during ethanol-induced hangover in mice and the involvement of adenosine receptors in this response. For this purpose, we tested the animals in the elevated plus maze 0.5–24 h after a single administration of ethanol (4 g/kg, i.p.). The elevated plus maze is one of the most widely used animal models of anxiety that has been pharmacologically and ethologically validated (Pellow et al, 1985; Lister, 1987). We also investigated the potential of adenosine and the selective adenosine receptor agonists CCPA (adenosine A1 receptor agonist) and N6-[2-(3,5-dimethoxyphenyl)-2-(2-methylphenyl)ethyl] adenosine (DPMA, adenosine A2A receptor agonist) to reduce anxiety-like behavior during ethanol hangover in mice.
MATERIALS AND METHODS
Subjects
A total of 476 male Swiss albino mice weighing 35–45 g from our own colony were used. They were kept in groups of 20 animals per cage and maintained in a room under controlled temperature (23±1°C). They were subjected to a 12-h light cycle (lights on 0700 h) with free access to food and water. All tests were carried out between 0900 and 1700 h. All procedures used in the present study complied with the guidelines on animal care of the UFSC Ethics Committee on the Use of Animals, which follows the ‘Principles of laboratory animal care’ from NIH publication no. 85–23.
Compounds
Ethanol (Merck, Brazil) was diluted in 0.9% NaCl (saline) to the concentration of 20% w/v. Adenosine (Sigma, USA), the selective adenosine A1 receptor agonist CCPA (Tocris, USA), and the selective adenosine A2A receptor agonist DPMA (Tocris, USA), together with the selective adenosine A1 receptor antagonist DPCPX (Tocris, USA), were dissolved in saline with 5% dimethylsulfoxide (DMSO). The control solution consisted of saline for ethanol and saline with 5% DMSO (vehicle) for adenosine, CCPA, DPMA, and DPCPX. All drug doses, selected according to previous literature (Jain et al, 1995; Florio et al, 1998; El Yacoubi et al, 2000; Prediger et al, 2004), were administered i.p. in a volume of 0.1 ml/10 g of body weight, 30 min before the elevated plus maze and open-field tests, except for the ethanol that was administered 0.5, 6, 12, 18, or 24 h before the experiments.
Elevated Plus Maze
The elevated plus maze was used on the basis of its documented ability to detect both anxiolytic- and anxiogenic-like drug effects in mice (Lister, 1987). Briefly, the apparatus was made of wood covered with impermeable Formica, and was placed 60 cm above the floor. The four arms were 18 cm long and 6 cm wide. Two opposite arms were surrounded by walls (6 cm high, closed arms), while the other two were devoid of enclosing walls (open arms). The four arms were connected by a central platform (6 × 6 cm). The experiments were conducted in a sound-attenuated room under low-intensity light (12 lux). Each subject was placed in the central area of the maze facing a closed arm. The animals were observed for a 5-min test period and anxiogenic-like effects were defined as a decrease in the proportion of open-arm entries divided by the total number of arm entries, and the time spent on open arms relative to the total time spent on both arms. Whenever a mouse placed all four paws onto an arm, one entry was recorded. The total number of closed-arm entries was utilized as a measure of locomotor activity.
Open Field
To discard the effects of treatment with the adenosine drugs in locomotor activity, the animals were placed for 5 min in an open-field arena (new environment). The apparatus, made of wood covered with impermeable Formica, had a black floor of 30 × 30 cm (divided by white lines into nine squares of 10 × 10 cm) and transparent walls, 15 cm high. The experiments were conducted in a sound-attenuated room under low-intensity light (12 lux). Each mouse was placed in the center of the open field and the number of total squares crossed and rearing were registered.
Blood Alcohol Concentrations
The onset of ethanol hangover symptoms in adult animals after acute exposure generally occurs when blood alcohol concentrations (BACs) approach or reach zero, and such symptoms can persist for several hours to a few days after ethanol has been metabolized from the body (Gauvin et al, 1992; Doremus et al, 2003; Varlinskaya and Spear, 2004). Thus, BACs were measured in separate groups of animals at 0.5, 2, 4, 6, and 12 h after a single i.p. injection of ethanol (4 g/kg), with the purpose of identifying appropriate intervals for assessment of ethanol aftereffects and, in later experiments, to determine the relationship between the pharmacokinetic variables and the magnitude and duration of hangover symptoms observed. BACs were determined by means of gas chromatography using Thermo Quest Autosystem apparatus equipped with a Tecmar 7000 headspace autosampler. Separation was achieved on a Poropack Q column under isothermal conditions (at 200°C). The temperature of the flame ionization detector (FID) was 230°C. A 0.5 ml sample of blood was mixed with 0.5 ml of a 1.6 g/l solution of n-propanolol used as an internal standard (IS). The samples were incubated in the autosamples for 7 min at 75°C. The mobile phase consisted of helium being delivered at a rate of 25 ml/min. Chromatograms were recorded and basic calculations were made using Chrom-card computer program.
Behavioral Procedures
Temporal properties of hangover-induced anxiety-like behavior in mice
To evaluate a possible time-dependent development of anxiety-like behavior during ethanol hangover in mice, independent groups of animals were tested in the elevated plus maze 0.5, 6, 12, 18, or 24 h after the i.p. administration of saline (control) or an acute dose of ethanol (4 g/kg). They were placed in the central area of the elevated plus maze, where their behavioral parameters (described above) were recorded over a period of 5 min.
Role of adenosine A1 and A2A receptors in hangover-induced anxiety-like behavior in mice
Firstly, to rule out effects per se of the adenosine agents tested in the anxiety-like behavior and locomotor activity of mice, we tested independent group of animals in the elevated plus maze and in the open-field arena 30 min after the acute administration of adenosine (1, 5, or 10 mg/kg, i.p.) or the selective adenosine receptor agonists CCPA (adenosine A1 receptor agonist, 0.05, 0.125, or 0.25 mg/kg, i.p.) or DPMA (adenosine A2A receptor agonist, 0.1, 1.0, or 5.0 mg/kg, i.p.), or the selective adenosine A1 receptor antagonist DPCPX (3.0 mg/kg, i.p.).
Then, we investigated the effect of acute administration of these same doses of adenosine (1, 5, or 10 mg/kg, i.p.), CCPA (0.05, 0.125, or 0.25 mg/kg, i.p.) or DPMA (0.1, 1.0, or 5.0 mg/kg, i.p.) or their vehicle (i.p.) on the anxiety-like behavior and locomotor activity during ethanol hangover in mice. The animals were submitted to elevated plus-maze or open-field performance 18 h (based on the previous experiment) after ethanol administration (4 g/kg, i.p.), and the adenosine receptor agonists were administered 30 min before the tests.
A subsequent experiment was performed to discover whether the current effects of CCPA on the hangover-induced anxiety-like behavior in mice were selective for the activation of adenosine A1 receptors. Thus, we administered a selected dose (based on our previous study: Prediger et al, 2004) of the adenosine A1 receptor antagonist DPCPX (3.0 mg/kg, i.p.) 15 min prior to CCPA injection (0.05 mg/kg, i.p.). The animals were submitted to elevated plus-maze performance 18 h after ethanol administration (4 g/kg, i.p.). DPCPX and CCPA were administered, respectively, 30 and 15 min before the test.
Statistical Analysis
All values are expressed as means±SEM. The statistical comparison of results was carried out using one- or two-way ANOVA, with treatment and time as independent variables. Dependent variables were the number of squares crossing and rearing (open field) as well as the percentages of entries and time spent on the open arms, and the number of enclosed arm entries (elevated plus maze). Following significant ANOVAs, multiple post hoc comparisons were performed using the Newman–Keuls test. The accepted level of significance for the tests was p⩽0.05. All tests were performed using the Statistica® software package.
RESULTS
Blood Alcohol Concentrations
Figure 1 displays the average plasma ethanol concentrations (in milligrams per deciliter) of adult male mice at each of five post-administration sampling intervals after 4 g/kg (i.p.) ethanol.
One-way ANOVA revealed a significant effect for the time factor (F4,26=187.43; p<0.0001). Post-hoc comparisons indicated that BACs dropped significantly at 4- and 6-h time points after ethanol injection. In addition, the estimated clearance times calculated from the regression equations revealed that the animals required approximately 8.5 h to clear the ethanol dose administered (4 g/kg, i.p.) (Figure 1).
Temporal Properties of Hangover-Induced Anxiety-Like Behavior in Mice
As illustrated in Figure 2, a time-dependent development of anxiety-like behavior was observed after the administration of an acute dose of ethanol (4 g/kg) in mice. Two-way ANOVA (treatment vs time) revealed a nonsignificant effect for the independent variables when analyzed alone. However, it indicated a significant effect for the interaction factor between treatment and time on the percentage of time spent on open arms (F4,62=2.85; p<0.05), the percentage of open-arm entries (F4,62=3.32; p<0.05), and the number of closed-arm entries (F4,62=5.82; p<0.001).
Subsequent Newman–Keuls tests indicated a significant (p⩽0.05) reduction in the percentages of entries and time spent on open arms at 12- and 18-h time points after the administration of ethanol (4 g/kg, i.p.), indicating an anxiogenic-like effect after ethanol administration at these times. Also, the group tested in the elevated plus maze 0.5 h after the administration of ethanol presented a significant reduction in locomotor activity compared to the control group, as shown by the reduced total number of closed-arm entries (Figure 2).
Role of Adenosine A1 and A2A Receptors in Hangover-Induced Anxiety-Like Behavior in Mice
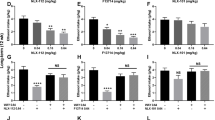
Table 1 summarizes the effects of the acute administration (30 min before the experiments) of adenosine (1, 5, or 10 mg/kg, i.p.) or the selective adenosine receptor agonists CCPA (adenosine A1 receptor agonist 0.05, 0.125, or 0.25 mg/kg, i.p.) or DPMA (adenosine A2A receptor agonist, 0.1, 1.0, or 5.0 mg/kg, i.p.), or the selective adenosine A1 receptor antagonist DPCPX (3.0 mg/kg, i.p.) in the elevated plus-maze and open-field tests in ‘nonintoxicated’ mice.
One-way ANOVA indicated a significant effect for treatment factor on the percentage of time spent on open arms (F10,70=6.35; p<0.0001), the percentage of open-arm entries (F10,70=4.44; p<0.0001), without significance for the number of closed-arm entries (F10,70=1.20; p=0.31). Post-hoc comparisons revealed that only the highest dose of CCPA tested (0.25 mg/kg, i.p.) significantly increased the mouse's exploration of the open arms of the maze, without significant change in the number of closed-arm entries, demonstrating an anxiolytic-like effect of CCPA at this dose (Table 1).
Furthermore, the treatment with the adenosine agents did not seem to interfere, at least at present doses, with the locomotor activity of the animals, since no alterations in total squares crossed (F10,67=2.44; p=0.32) and rearing (F10,67=1.20; p=0.31) were observed in the open-field arena (Table 1).
Indeed, based on the previous experiment where the acute ethanol withdrawal-induced anxiety was most pronounced at 18 h after ethanol administration, we selected the same post-injection time to test the effect of the adenosine receptor agonists. The effects of adenosine (1, 5, or 10 mg/kg, i.p.), administered 30 min before the elevated plus-maze performance, are illustrated in Figure 3. Adenosine administration promoted a significant effect (ANOVA) on the percentage of time spent on open arms (F3,24=16.73; p<0.0001) and the percentage of open-arm entries (F3,24=92.63; p<0.001), without significance for the number of closed-arm entries (F3,24=1.14; p=0.35) (Figure 3).
Effect of treatment with adenosine on the anxiety-like behavior during ethanol hangover in mice. Adenosine (1, 5, or 10 mg/kg, i.p.) or vehicle (i.p.) was injected 18 h after ethanol challenge (4 g/kg, i.p.), and 30 min later the animals were tested in the elevated plus maze. Each bar represents the mean±SEM of seven animals per group. *p⩽0.05 compared to the control-treated group (Newman–Keuls test).
Subsequent Newman–Keuls tests indicated that adenosine, at doses of 5 and 10 mg/kg (i.p.), produced an increase in the exploration of the open arms of the elevated plus maze, with no change in the number of closed-arm entries, indicating that the activation of adenosine receptors was able to reduce the anxiety-like behavior during ethanol hangover in mice (Figure 3).
Figure 4 shows the effects of the selective adenosine A1 receptor agonist CCPA (0.05, 0.125, or 0.25 mg/kg, i.p.) on the ethanol hangover-induced anxiety in mice. One-way ANOVA indicated a significant effect for treatment factor on the percentage of time spent on open arms (F3,24=13.46; p<0.0001), the percentage of open-arm entries (F3,24=13.05; p<0.0001), and the number of closed-arm entries (F3,24=9.51; p<0.001) (Figure 4).
Effect of treatment with the adenosine A1 receptor agonist CCPA on the anxiety-like behavior during ethanol hangover in mice. CCPA (0.05, 0.125, or 0.25 mg/kg, i.p.) or vehicle (i.p.) was injected 18 h after ethanol challenge (4 g/kg, i.p.), and 30 min later the animals were tested in the elevated plus maze. Each bar represents the mean±SEM of seven animals per group. *p⩽0.05 compared to the control-treated group (Newman–Keuls test).
Post-hoc comparisons indicated that CCPA, at doses of 0.05 and 0.125 mg/kg (i.p.), produced a selective increase in the exploration of the open arms of the elevated plus maze, with no change in the frequency of closed-arm entries. Also, further comparisons between groups revealed that the highest dose of CCPA tested (0.25 mg/kg, i.p.) significantly decreased the locomotor activity of mice compared to the control group, as shown by the reduced total number of closed-arm entries (Figure 4).
As can be seen from Figure 5, in contrast to the results obtained with adenosine and CCPA, the administration of the adenosine A2A receptor agonist DPMA (0.1, 1.0, or 5.0 mg/kg, i.p.) did not significantly alter the behavioral parameters in the elevated plus maze: percentage of time spent on open arms (F3,24=0.86; p=0.47); percentage of open-arm entries (F3,24=0.11; p=0.95) and number of closed-arm entries (F3,24=0.05; p=0.98) (Figure 5).
Effect of treatment with the adenosine A2A receptor agonist DPMA on the anxiety-like behavior during ethanol hangover in mice. DPMA (0.1, 1.0, or 5.0 mg/kg, i.p.) or vehicle (i.p.) was injected 18 h after ethanol challenge (4 g/kg, i.p.) and 30 min later the animals were tested in the elevated plus maze. Each bar represents the mean±SEM of seven animals per group.
The effects of the administration of the adenosine A1 receptor antagonist DPCPX (3.0 mg/kg, i.p.) 15 min prior to the injection of CCPA (0.05 mg/kg, i.p.) in the hangover-induced anxiety are illustrated in Figure 6. One-way ANOVA revealed a significant effect for the treatment factor in the percentage of time spent on open arms (F3,21=21.74; p<0.0001) and number of open-arm entries (F3,21=40.12; p<0.0001), without significance for the number of closed-arm entries (F3,21=0.18; p=0.91) (Figure 6).
Effect of the administration of the adenosine A1 receptor antagonist DPCPX (3.0 mg/kg, i.p.), 15 min prior to the injection of CCPA (0.05 mg/kg, i.p.) or vehicle (i.p.) on mice previously (18 h before) treated with ethanol (4 g/kg, i.p.), and tested 30 min later in the elevated plus maze. Each bar represents the mean±SEM of 6–7 animals per group. *p⩽0.05 compared to the control-treated group (Newman–Keuls test).
Post-hoc comparisons showed that the dose of DPCPX tested did not significantly alter the behavioral parameters in the elevated plus maze when it was administered alone. However, this same dose was able to reverse the anxiolytic-like effect induced by CCPA during the ethanol hangover, reinforcing the selective effect of CCPA at adenosine A1 receptors in the present results (Figure 6).
Furthermore, as shown in Table 2, the effects induced by adenosine and CCPA administration in increasing the mouse's exploration of the open arms of the elevated plus maze during ethanol hangover did not seem related to motor impairments, since no alterations in total squares crossed and rearing were observed in the open field during ethanol hangover, except for the highest dose of CCPA tested (0.25 mg/kg, i.p.) (Table 2).
DISCUSSION
The present findings demonstrate a time-dependent development of anxiety-like behavior after the i.p. administration of a single dose of ethanol (4 g/kg) in mice, as indicated by a significant reduction in the exploration of the open arms in the elevated plus maze. This acute ethanol withdrawal-induced anxiety was most pronounced at 18 h after ethanol administration, in the time in which ethanol was completely cleared from the mice blood. More importantly, our results have demonstrated for the first time that the activation of adenosine A1 receptors, but not adenosine A2A receptors, reduces the anxiogenic-like behavior observed during acute ethanol withdrawal in mice.
The negative signs from ethanol withdrawal, such as nausea, tremors, tachycardia, irritability, and anxiety, are frequently verified early in the withdrawal period following extended periods of alcohol consumption in both humans and rodents (for a review, see Finn and Crabbe, 1997), and they represent an important motivational factor for ethanol relapse (Cloninger, 1987). On the other hand, alcoholism and alcohol abuse often involve heavy bouts of binge-like drinking interspersed with periods of abstinence, while some signs of withdrawal (such as fatigue, tremor, anxiety and depression) may be observed even after acute ingestion of substantial amounts of alcohol—a phenomenon commonly referred to as acute ethanol withdrawal or hangover (Smith and Barnes, 1983; Bogin et al, 1986; Wiese et al, 2000). Although many studies have consistently demonstrated increases in anxiety-like behavior during the withdrawal period after chronic exposure to ethanol in rodents (File et al, 1991, 1993; Lal et al, 1991; Knapp et al, 1993; Gatch et al, 1999; Gatch and Lal, 2001), there are limited experimental findings regarding this symptom after a single ethanol challenge dose.
In the current study, adult male mice showed a clear time-dependent development of anxiogenic-like profile following the administration of an acute dose of ethanol (4 g/kg, i.p.), as indicated by a reduced frequency of entries and the time spent in the open arms of the elevated plus maze. This response was most pronounced in the interval between 12 and 18 h after ethanol administration, which coincides with the time required for complete ethanol clearance. Although the elevated plus maze has been suggested to be an ‘ethologically’ valid animal model of human anxiety (Dawson and Tricklebank, 1995), a major difficulty is to determine a specific form of clinical anxiety that can be associated with a particular animal model. As proposed by Lister (1990), behavioral responses evaluated in tests such as the elevated plus maze, which include a temporary anxiety-inducing situation, are thought to reflect the transient states of anxiety rather than a chronic anxiety-related trait. Regardless of anxiety definition, it is important to mention that these results are in accordance with previous studies that have demonstrated an increase in anxiety-like behavior during ethanol hangover in adult male rats assessed in the startle-induced ultrasonic vocalization (Brasser and Spear, 2002), elevated plus maze (Doremus et al, 2003), and social interaction (Varlinskaya and Spear, 2004) tests.
In addition, an increasing amount of evidence suggests that adenosine receptors mediate important actions of ethanol in both humans and rodents. Acute exposure to ethanol inhibits adenosine re-uptake via a facilitative nucleoside transporter, increasing the extracellular concentration of adenosine (Nagy et al, 1990; Krauss et al, 1993). In humans, the nonselective adenosine receptor antagonist caffeine reduces sleepiness and psychomotor performance impairment produced by moderate-to-high ethanol doses (Franks et al, 1975; Fillmore and Vogel-Sprott, 1995; Liguori and Robinson, 2001; Drake et al, 2003). In rodents, adenosine receptors seem to modulate some of the pharmacological properties of ethanol, such as sedative/hypnotic effects (El Yacoubi et al, 2003), motor incoordination (Meng and Dar, 1995; Barwick and Dar, 1998; Dar, 2001), and development of rapid tolerance to ethanol-induced motor impairments (Batista et al, 2005).
In the current study, we have presented evidence that the acute administration of ‘nonanxiolytic’ doses of adenosine (5–10 mg/kg, i.p.) or the selective adenosine A1 receptor agonist CCPA (0.05–0.125 mg/kg, i.p.), but not the adenosine A2A receptor agonist DPMA (0.1–5.0 mg/kg, i.p.), reduces the anxiety-like behavior during ethanol hangover in mice, as indicated by a significant increase in the exploration of the open arms of the elevated plus maze. The present effects of adenosine and CCPA administration cannot be explained by a locomotion deficit of the animals, since it was not observed in the total number of closed-arm entries in the plus maze as well as in the behavioral parameters evaluated in the open-field arena. Moreover, this response seems to be specifically dependent on the activation of adenosine A1 receptors, since the effect of CCPA (0.05 mg/kg, i.p.) on hangover-induced anxiety-like behavior was prevented by the pretreatment with the selective adenosine A1 receptor antagonist DPCPX (3.0 mg/kg, i.p.). It is noteworthy that in our previous study the same dose of DPCPX, ineffective per se in the elevated plus maze, was able to block the anxiolytic-like actions of ethanol (1.2 g/kg, i.p.) in this paradigm (Prediger et al, 2004). Although we did not measure plasma or brain ethanol concentrations in the animals treated with adenosine and CCPA, a possible pharmacokinetic interaction between these drugs and ethanol cannot be applied to explain the present results, since they were administered to the animals 18 h after ethanol (4.0 g/kg, i.p.) injection, and at this time the ethanol had already been eliminated. Therefore, adenosine and CCPA appear to specifically reduce the anxiety-like behavior during ethanol hangover, without causing other effects per se that could interfere with the interpretation of the results. However, additional studies using other selective drugs towards adenosine A2A receptors are necessary in order to discard completely the involvement of this receptor subtype in ethanol hangover. Previous studies have demonstrated the participation of these receptors in ‘anxiety’ responses (Ledent et al, 1997), and the potential of adenosine A2A receptor agonists in reducing some signs from chronic ethanol withdrawal (Kaplan et al, 1999) in rodents.
Consistent with the present data, an increasing amount of evidence have indicated that adenosine A1 receptors modulate anxiety-like responses in mice, with adenosine A1 receptor knockout mice displaying enhanced anxiety (Johansson et al, 2001; Gimenez-Llort et al, 2002; Lang et al, 2003), while adenosine A1 receptor agonists present anxiolytic properties (Florio et al, 1998). Moreover, we have recently demonstrated that the anxiolytic-like effect induced by ethanol in mice is modulated by the activation of adenosine A1 receptors (but not by A2A receptors), since this response is blocked by the previous administration of caffeine or DPCPX, and is enhanced following the combined administration of ethanol with CCPA (Prediger et al, 2004). The present results replicate and extend the findings obtained by other authors (Concas et al, 1994; Kaplan et al, 1999) who have tested the potential of adenosine A1 receptor agonist treatment in suppressing signs of withdrawal in rodents after chronic exposure to ethanol. Concas et al (1994) have demonstrated that CCPA produces inhibition of withdrawal symptoms, such as tremors and audiogenically induced seizures, in rats treated repeatedly with ethanol (12–18 g/kg daily for 6 days), an effect prevented by DPCPX. Similar results have been reported by Kaplan et al (1999) in mice receiving a 14-day liquid diet containing ethanol and treated with the adenosine A1 receptor agonist R-PIA during the withdrawal period.
Although the exact anatomical sites and molecular mechanisms involved in the mediation of adenosine receptors effects in ethanol hangover are not known, a speculative hypothesis can be given. Previous studies have demonstrated that adenosine A1 receptor agonists can inhibit the neural release of glutamate (Dolphin and Prestwich, 1985; Fredholm et al, 1989) and acetylcholine (Jin et al, 1993; Ribeiro et al, 1996), two excitatory neurotransmitters that have been implicated as playing an important role in the ethanol withdrawal syndrome (Rossetti and Carboni, 1995; Imperato et al, 1998). Thus, this mechanism may underlie the present effectiveness of adenosine and CCPA in reducing acute ethanol withdrawal-induced anxiety.
Additionally, a role for adenosine A1 receptors in mediating ethanol's withdrawal effects has been suggested from radioligand-binding studies. Withdrawal after chronic exposure to ethanol upregulates adenosine A1-binding sites in cerebellar (Concas et al, 1996) and cerebral cortex tissues (Daly et al, 1994) in rodents. After chronic ethanol vapor exposure and after single and repeated withdrawal episodes, mice demonstrate increases in cortical adenosine A1-binding sites without changes in striatal adenosine A2A receptors (Jarvis and Becker, 1998). Taken together, these findings indicate that the increased expression of adenosine A1 receptors, a receptor usually coupled to ‘inhibitory’ G-proteins (Gi and Go), seems to be an important adaptive response to counteract the deleterious effects during ethanol withdrawal. Although hangover effects are thought to reflect a milder precursor of similar symptoms observed after withdrawal from chronic ethanol treatment (Freund, 1980), little is known about the ontogenetic differences in either acute or chronic ethanol withdrawal. Indeed, although we did not present data relating density of adenosine A1 and A2A-receptor binding sites in the brain tissues of mice during ethanol hangover, behavioral observations indicate that, similar to what was verified during withdrawal from chronic ethanol exposure, an increased expression of adenosine A1 receptors might occur during acute withdrawal. Withdrawing mice demonstrated an increased sensitivity to the sedative effects of the adenosine A1 receptor agonist CCPA, indicated by a significant reduction in the locomotor activity (verified in both elevated plus-maze and open-field tests) at the dose of 0.25 mg/kg (i.p.), while this sedative effect was detected in nonwithdrawing mice only at higher CCPA doses (0.5–1.0 mg/kg, i.p.) (Prediger et al, 2004).
It is important to emphasize that adenosine mechanisms seem to be involved in the expression of withdrawal signs of drugs other than ethanol (eg, opiate and cocaine). For example, most studies agree that adenosine A1 and A2A receptor agonists attenuate while adenosine receptor antagonists exacerbate morphine-induced withdrawal signs (such as ‘wet-dog’ shakes, diarrhoea, teeth chattering, jumping, and writhing) of mice (Kaplan and Sears, 1996; Zarrindast et al, 1999) and rats (Salem and Hope, 1997). In view of these considerations, it seems clear that the endogenous adenosine is released during withdrawal from different drugs and acts at its receptors to produce an ongoing inhibitory ‘tone’ (reducing the specific withdrawal signs associated with each drug) which, when blocked, results in the enhancement of withdrawal behavior.
In conclusion, the present findings demonstrate that adult male mice manifest anxiogenic-like behavior following a single ethanol (4.0 g/kg, i.p.) administration, that is acute ethanol withdrawal or hangover. More importantly, our results reinforce the hypothesis of the involvement of adenosine receptors in the anxiety-like responses, and show for the first time that the activation of adenosine A1 receptors, but not A2A receptors, reduces the hangover-related anxiety responses in mice. The advantage of adenosine agents over benzodiazepines (the drug of choice for the suppression of ethanol withdrawal) may relate to their improved side-effects profile (such as low abuse potential), but, certainly, additional studies are warranted to better evaluate the potential of adenosine receptor agonists in the treatment of ethanol withdrawal.
References
Alsene K, Deckert J, Sand P, de Wit H (2003). Association between A2a receptor gene polymorphisms and caffeine-induced anxiety. Neuropsychopharmacology 28: 1694–1702.
Barwick VS, Dar MS (1998). Adenosinergic modulation of ethanol-induced motor incoordination in the rat motor cortex. Prog Neuropsychopharmacol Biol Psychiatry 22: 587–607.
Batista LC, Prediger RDS, Morato GS, Takahashi RN (2005). Blockade of adenosine and dopamine receptors inhibits the development of rapid tolerance to ethanol in mice. Psychopharmacology 181: 714–721.
Bogin RM, Nostrant TT, Young MJ (1986). Propranolol for the treatment of the alcoholic hangover. Am J Drug Alcohol Abuse 12: 279–284.
Brasser SM, Spear NE (2002). Physiological and behavioral effects of acute ethanol hangover in juvenile, adolescent, and adult rats. Behav Neurosci 116: 305–320.
Cloninger CR (1987). Neurogenetic adaptive mechanisms in alcoholism. Science 236: 410–416.
Commissaris RL, McCloskey TC, Damian GM, Brown BD, Barraco RA, Altman HJ (1990). Antagonism of the anti-conflict effects of phenobarbital, but not diazepam, by the A-1 adenosine agonist 1-PIA. Psychopharmacology 102: 283–290.
Concas A, Cuccheddu T, Floris S, Mascia MP, Biggio G (1994). 2-Chloro-N6-cyclopentyladenosine (CCPA), an adenosine A1 receptor agonist, suppressed ethanol withdrawal syndrome in rats. Alcohol Alcohol 29: 261–264.
Concas A, Mascia MP, Cucceddhu T, Floris S, Mostallino MC, Perra C et al (1996). Chronic ethanol intoxication enhances [3H]-CCPA binding and does not reduce A1 adenosine receptor function in rat cerebellum. Pharmacol Biochem Behav 53: 249–255.
Cunha RA (2001). Adenosine as a neuromodulator and as a homeostatic regulator in the nervous system: different roles, different sources and different receptors. Neurochem Int 38: 107–125.
Daly JW, Shi D, Wong V, Nikodijevic O (1994). Chronic effects of ethanol on central adenosine function of mice. Brain Res 650: 153–156.
Dar MS (2001). Modulation of ethanol-induced motor incoordination by mouse striatal A1 adenosinergic receptor. Brain Res Bull 55: 513–520.
Dawson GR, Tricklebank MD (1995). Use of the elevated plus-maze in the search for novel anxiolytic agents. Trends Pharmacol Sci 16: 33–36.
Dolphin AC, Prestwich SA (1985). Pertussis toxin reverses adenosine inhibition of neuronal glutamate release. Nature 316: 148–150.
Doremus TL, Brunell SC, Varlinskaya EI, Spear LP (2003). Anxiogenic effects during withdrawal from acute ethanol in adolescent and adult rats. Pharmacol Biochem Behav 75: 411–418.
Drake CL, Roehrs T, Turner L, Scofield HM, Roth T (2003). Caffeine reversal of ethanol effects on the multiple sleep latency test, memory, and psychomotor performance. Neuropsychopharmacology 28: 371–378.
El Yacoubi M, Ledent C, Parmentier M, Costentin J, Vaugeois JM (2000). The anxiogenic-like effect of caffeine in two experimental procedures measuring anxiety in the mouse is not shared by selective A2A adenosine receptor antagonists. Psychopharmacology 148: 153–163.
El Yacoubi M, Ledent C, Parmentier M, Costentin J, Vaugeois JM (2003). Caffeine reduces hypnotic effects of alcohol through adenosine A2A receptor blockade. Neuropharmacology 45: 977–985.
File SE, Andrews N, Al-Farhan M, Wu PY (1993). The role of 5-HT in the anxiogenic effects of acute ethanol withdrawal and in the long-lasting cognitive deficits. Alcohol Alcohol (Suppl) 2: 495–499.
File SE, Hyde J, Pool M (1976). Effects of ethanol and chlordiazepoxide on social interaction in rats. Br J Pharmacol 58: 465.
File SE, Zharkovsky A, Gulati K (1991). Effects of baclofen and nitrendipine on ethanol withdrawal responses in the rat. Neuropharmacology 30: 183–190.
Fillmore MT (2003). Alcohol tolerance in humans is enhanced by prior caffeine antagonism of alcohol-induced impairment. Exp Clin Psychopharmacol 11: 9–17.
Fillmore MT, Vogel-Sprott M (1995). Behavioral effects of combining alcohol and caffeine: the contribution of drug-related expectancies. Exp Clin Psychopharmacol 3: 33–38.
Finn DA, Crabbe JC (1997). Exploring alcohol withdrawal syndrome. Alcohol Health Res World 21: 149–156.
Florio C, Prezioso A, Papaioannou A, Vertua R (1998). Adenosine A1 receptors modulate anxiety in CD1 mice. Psychopharmacology 136: 311–319.
Franks HM, Hagedorn H, Hensley VR, Hensley WJ, Starmer GA (1975). The effect of caffeine on human performance, alone and in combination with ethanol. Psychopharmacology 45: 177–181.
Fredholm BB, Proctor W, Van der Ploeg I, Dunwiddie TV (1989). In vivo pertussis toxin treatment attenuates some, but not all, adenosine A1 effects in slices of the rat hippocampus. Eur J Pharmacol 172: 249–262.
Fredholm BB, Ijzerman AP, Jacobson KA, Klotz KN, Linden J (2001). International Union of Pharmacology: XXV. Nomenclature and classification of adenosine receptors. Pharmacol Rev 53: 527–552.
Freund G (1980). Comparison of alcohol dependence, withdrawal, and hangover in humans and animals. In: Eriksson K, Sinclair JD, Kiianmaa K (eds). Animal Models in Alcohol Research. Academic Press: New York. pp 293–308.
Gatch MB, Lal H (2001). Animal models of the anxiogenic effects of ethanol withdrawal. Drug Dev Res 54: 95–115.
Gatch MB, Wallis CJ, Lal H (1999). Effects of NMDA antagonists on ethanol withdrawal induced ‘anxiety’ in the elevated plus maze. Alcohol 19: 207–211.
Gauvin DV, Goulden KL, Holloway FA (1997). The paradoxical hedonic valence of acute ethanol withdrawal (hangover) states in rats: place and taste conditioning. Alcohol 14: 261–268.
Gauvin DV, Goulden KL, Holloway FA (1993). State-dependent stimulus control: cueing attributes of ethanol ‘hangover’ in rats. Alcohol Clin Exp Res 17: 1210–1214.
Gauvin DV, Youngblood BD, Holloway FA (1992). The discriminative stimulus properties of acute ethanol withdrawal (hangover) in rats. Alcohol Clin Exp Res 16: 336–341.
Gimenez-Llort L, Fernandez-Teruel A, Escorilhuela RM, Fredholm BB, Tobena A, Pekny M et al (2002). Mice lacking the adenosine A1 receptor are anxious and aggressive, but are normal learners with reduced muscle strength and survival rate. Eur J Neurosci 16: 547–550.
Hamilton SP, Slager SL, De Leon AB, Heiman GA, Klein DF, Hodge SE et al (2004). Evidence for genetic linkage between a polymorphism in the adenosine 2A receptor and panic disorder. Neuropsychopharmacology 29: 558–565.
Imperato A, Dazzi L, Carta G, Colombo G, Biggio G (1998). Rapid increase in basal acetylcholine release in the hippocampus of freely moving rats induced by withdrawal from long-term ethanol intoxication. Brain Res 784: 347–350.
Jain N, Kemp N, Adeyemo O, Buchanan P, Stone TW (1995). Anxiolytic activity of adenosine receptor activation in mice. Br J Pharmacol 116: 2127–2133.
Jarvis MF, Becker HC (1998). Single and repeated episodes of ethanol withdrawal increase adenosine A1, but not A2A, receptor density in mouse brain. Brain Res 786: 80–88.
Jin S, Johansson B, Fredholm BB (1993). Effects of adenosine A1 and A2 receptor activation on electrically evoked dopamine and acetylcholine release from rat striatal slices. J Pharmacol Exp Ther 267: 801–808.
Johansson B, Halldner L, Dunwiddie TV, Masino SA, Poelchen W, Gimenez-Llort L et al (2001). Hyperalgesia, anxiety, and decreased hypoxic neuroprotection in mice lacking the adenosine A1 receptor. Proc Natl Acad Sci USA 98: 9407–9412.
Kaplan GB, Bharmal NH, Leite-Morris KA, Adams WR (1999). Role of adenosine A1 and A2A receptors in the alcohol withdrawal syndrome. Alcohol 19: 157–162.
Kaplan GB, Sears MT (1996). Adenosine receptor agonists attenuate and adenosine receptor antagonists exacerbate opiate withdrawal signs. Psychopharmacology 123: 64–70.
Knapp DJ, Saiers JA, Pohorecky LA (1993). Observations of novel behaviors as indices of ethanol withdrawal-induced anxiety. Alcohol Alcohol (Suppl) 2: 489–493.
Krauss SW, Ghirnikar RB, Diamond I, Gordon AS (1993). Inhibition of adenosine uptake by ethanol is specific for one class of nucleoside transporters. Mol Pharmacol 44: 1021–1026.
Lal H, Prather PL, Rezazadeh SM (1991). Anxiogenic behavior in rats during acute and protracted ethanol withdrawal: reversal by buspirone. Alcohol 8: 467–471.
Lang UE, Lang F, Richter K, Vallon V, Lipp HP, Schnermann J et al (2003). Emotional instability but intact spatial cognition in adenosine receptor 1 knockout mice. Behav Brain Res 145: 179–188.
Ledent C, Vaugeois JM, Schiffmann SN, Pedrazzini T, El Yacoubi M, Vanderhaeghen JJ et al (1997). Aggressiveness, hypoalgesia and high blood pressure in mice lacking the adenosine A2a receptor. Nature 388: 674–678.
Liguori A, Robinson JH (2001). Caffeine antagonism of alcohol-induced driving impairment. Drug Alcohol Depend 63: 123–129.
Lister RG (1987). The use of a plus-maze to measure anxiety in the mouse. Psychopharmacology 92: 180–185.
Lister RG (1990). Ethologically-based animal models of anxiety disorders. Pharmacol Ther 46: 321–340.
Meng ZH, Dar MS (1995). Possible role of striatal adenosine in the modulation of acute ethanol-induced motor incoordination in rats. Alcohol Clin Exp Res 19: 892–901.
Millan MJ (2003). The neurobiology and control of anxious states. Prog Neurobiol 70: 83–244.
Morse AC, Schulteis G, Holloway FA, Koob GF (2000). Conditioned place aversion to the ‘hangover’ phase of acute ethanol administration in the rat. Alcohol 22: 19–24.
Nagy LE, Diamond I, Casso DJ, Franklin C, Gordon AS (1990). Ethanol increases extracellular adenosine by inhibiting adenosine uptake via the nucleoside transporter. J Biol Chem 265: 1946–1951.
Pellow S, Chopin P, File SE, Briley M (1985). Validation of open: closed arm entries in an elevated plus-maze as a measure of anxiety in the rat. J Neurosci Methods 14: 149–167.
Prediger RDS, Batista LC, Takahashi RN (2004). Adenosine A1 receptors modulate the anxiolytic-like effect of ethanol in the elevated plus-maze in mice. Eur J Pharmacol 499: 147–154.
Ribeiro JA, Cunha RA, Correia-de-Sa P, Sebastiao AM (1996). Purinergic regulation of acetylcholine release. Prog Brain Res 109: 231–241.
Rossetti ZL, Carboni S (1995). Ethanol withdrawal is associated with increased extracellular glutamate in the rat striatum. Eur J Pharmacol 283: 177–183.
Salem A, Hope W (1997). Effect of adenosine receptor agonists and antagonists on the expression of opiate withdrawal in rats. Pharmacol Biochem Behav 57: 671–679.
Smith CM, Barnes GM (1983). Signs and symptoms of hangover: prevalence and relationship to alcohol use in a general adult population. Drug Alcohol Depend 11: 249–269.
Varlinskaya EI, Spear LP (2004). Acute ethanol withdrawal (hangover) and social behavior in adolescent and adult male and female Sprague–Dawley rats. Alcohol Clin Exp Res 28: 40–50.
Wiese JG, Shlipak MG, Browner WS (2000). The alcohol hangover. Ann Intern Med 132: 897–902.
Zarrindast MR, Naghipour B, Roushan-zamir F, Shafaghi B (1999). Effects of adenosine receptor agents on the expression of morphine withdrawal in mice. Eur J Pharmacol 369: 17–22.
Acknowledgements
This work was supported by the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq)-Brazil. We are grateful to Dr Lourival Abreu Júnior for his expert assistance in blood alcohol measurements. RDSP and LCB receive scholarships from CNPq-Brazil. RNT is a CNPq research fellow. We also thank Professor Giles A Rae and the anonymous reviewers for helpful comments on an earlier draft of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Prediger, R., da Silva, G., Batista, L. et al. Activation of Adenosine A1 Receptors Reduces Anxiety-Like Behavior During Acute Ethanol Withdrawal (Hangover) in Mice. Neuropsychopharmacol 31, 2210–2220 (2006). https://doi.org/10.1038/sj.npp.1301001
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.npp.1301001
Keywords
This article is cited by
-
Cortical astrocytes regulate ethanol consumption and intoxication in mice
Neuropsychopharmacology (2021)
-
Mechanisms of the psychostimulant effects of caffeine: implications for substance use disorders
Psychopharmacology (2016)
-
Abstinence following Alcohol Drinking Produces Depression-Like Behavior and Reduced Hippocampal Neurogenesis in Mice
Neuropsychopharmacology (2009)