Abstract
Immobilization stress upregulates c-Fos expression in several CNS areas. Repeated stress or the use of drugs can modulate stress-induced c-Fos expression. Here, we investigated in 40 different areas of the rat brain the effects of dexamethasone (SDX, a synthetic glucocorticoid), diazepam (SBDZ, a benzodiazepine), and imipramine (IMI, an antidepressant) on the c-Fos expression induced by restraint stress. Wistar rats were divided into four groups and submitted to 20 days of daily injection of saline (three first groups) or imipramine, 15 mg/kg, i.p. On day 21, animals were submitted to injections of saline (somatosensory, SS), SDX (1 mg/kg, i.p.), SBDZ (5 mg/kg, i.p.), or IMI (15 mg/kg, i.p.) before being submitted to restraint. Immediately after stress, the animals were perfused and their brains processed with immunohistochemistry for c-Fos (Ab-5 Oncogene Science). Dexamethasone reduced stress-induced c-Fos expression in SS cortex, hippocampus, paraventricular nucleus of the hypothalamus (PVH), and locus coeruleus (LC), whereas diazepam reduced c-Fos staining in the SS cortex, hippocampus, bed nucleus of stria terminalis, septal area, and hypothalamus (preoptic area and supramammillary nucleus). Chronic administration of imipramine decreased staining in the hippocampus, PVH, and LC, while increasing it in the nucleus raphe pallidus. We conclude that dexamethasone, diazepam and imipramine differentially modulate stress-induced Fos expression. The present study provides an important comparative background that may help in the further understanding of the effects of these compounds and on the brain activation as well as on the behavioral, neuroendocrine, and autonomic responses to stress.
Similar content being viewed by others
INTRODUCTION
The expression of immediate-early genes (IEGs) corresponds to the activation of specific circuitry of the brain related to perception and integration of primary stimuli as well as to neuroendocrine, autonomic, and behavioral responses. Immobilization stress for 30 min can induce intense expression of c-Fos (and other IEGs) in several brain areas such as alocortex and neocortex, lateral septal area, paraventricular and dorsomedial nuclei of the hypothalamus, retrochiasmatic area, medial and cortical nuclei of the amygdala, periaqueductal gray matter, and locus coeruleus (LC) (Cullinan et al, 1995). This widespread and intense activation of CNS has been related to the richness and complexity of the stress response. Stress represents a complex orchestrated response to a stimulus that may be coded at motor, sensory, autonomic, and cognitive levels, and the activation of hypothalamic-pituitary-adrenal (HPA) axis and of structures of autonomic output represents but one of the primary manifestation of these responses (Cullinan et al, 1995). The activation of different brain structures can be part of facilitatory or inhibitory circuitry on the HPA axis (Lachuer et al, 1994; Li and Sawchenko, 1998; Herman et al, 2003).
Drugs, therapies, and behavioral procedures can alter the neuronal activation induced by stress. Stress-induced c-Fos expression can be modulated by different drug classes such as antidepressants, anxiolytics, and glucocorticoids. Dexamethasone (a synthetic glucocorticoid) can reduce the immobilization-induced expression of c-Fos RNAm in the paraventricular nucleus of the hypothalamus (PVH) (Imaki et al, 1995a). Benzodiazepines exert many effects, which oppose those of corticotropin-releasing factor (CRF), including anxiolysis and suppression of activation of the pituitary–adrenal axis induced by stress (Imaki et al, 1995b). Diazepam, a benzodiazepine, inhibits the immobilization-induced c-Fos expression in the brain cortex and hippocampus of rats (Bozas et al, 1997). Antidepressant therapies, such as the chronic administration of electroconvulsive seizures, reduce the c-Fos expression induced by stress in the rat frontal cortex (Morinobu et al, 1995). A similar effect occurs after chronic administration of antidepressants, as imipramine, sertraline, tranilcipromine, and desipramine (Morinobu et al, 1995).
There is considerable evidence on the clinical and behavioral effects of psychoactive agents; however, there are still many open questions with regard to the effects of these drugs on stress induced of brain activation. Furthermore, when available, these studies most often assess the effects of different agents only in a restricted number of brain structures. Here, we evaluated three different prototypic compounds, dexamethasone (a synthetic glucocorticoid), diazepam (a benzodiazepine), and imipramine (an antidepressant) in the immobilization-induced c-Fos expression over 40 different brain structures.
MATERIALS AND METHODS
Subjects
Adult, male, Wistar, albino rats, weighing 180–230 g, from the local breeding facilities (CEDEME-UNIFESP) were used in the present study. Animals were kept under conditions of controlled temperature (23±2°C) and illumination (12/12 h cycle, lights on at 0700 and had free access to water and standard rat chow diet (Nuvilab®). All experimental protocols were approved by the Animal Care and Use Committee of UNIFESP being in accordance with NIH guidelines on animal care. Experiments were carried out between 0900 and 1200.
EXPERIMENTAL DESIGN
Animals were then distributed into one of four groups (Figure 1) as follows:
Somatosensory (SS) group (n=5): animals were submitted to 20 days of daily injection of saline and on the 21st day to the injection of saline 60 min before the restraint stress.
SDX group (n=5): animals were submitted to 20 days of daily injection of saline and on the 21st day to the injection of dexamethasone (1 mg/kg, i.p.) (Unlap and Jope, 1994) 60 min before the restraint stress.
SBDZ group (n=5): animals were submitted to 20 days of daily injection of saline and on the 21st day to the injection of diazepam (5 mg/kg, i.p.) (Beck and Fibiger, 1995) 40 min before the restraint stress.
IMI group (n=5): animals were submitted to 20 days of daily injection of imipramine (15 mg/kg, i.p.) (Morinobu et al, 1995) and on the 21st day to the injection of imipramine (15 mg/kg, i.p.) 60 min before the restraint stress.
The time of treatment for the IMI group (20 days) was based on the previous results demonstrating the need of a chronic treatment with IMI for obtaining an antidepressant effect (Morinobu et al, 1995). Based on the time for peak plasma concentration of each drug, the time of drug injection before the restraint was 40 min for the SBDZ group and 60 min for the other groups.
For the restraint, rats were placed in a plastic cylinder for 60 min. Immediately after the period of restraint, stress animals were anesthetized (Thiopental, 50 mg/kg) and perfused transcardially with saline followed by 4% paraformaldehyde in KPBS (pH 6.8) at 4°C.
Immunohistochemistry
Brains were removed immediately after perfusion, stored overnight in sucrose 30%, and the sections (32 μm) were cut coronally on a cryostat. The sections were processed for the immunohistochemical detection of c-Fos protein using a conventional avidin–biotin–immunoperoxidase technique to localize an antiserum raised against a synthetic N-terminal fragment of human Fos protein (Ab-5, Oncogene Sciences). Briefly, free-floating sections were pretreated with hydrogen peroxidase for 10 min. Sections were treated with normal goat serum (1:100) and 0.3% Triton X-100 for 2 h and incubated with the primary antiserum at a dilution 1:10 000 in KPBS at room temperature for 20 h. Subsequently, the sections were incubated with a secondary antibody (goat anti-rabbit IgG 1:200—Vector) for 90 min at room temperature and treated with avidin–biotin complex (Vector 1:100) for 90 min. Sections were submitted to nickel-intensified diaminobenzidine reaction. Between steps, the sections were rinsed in KPBS (pH 6.8) 0.05 M. The tissue was agitated on a rotator between each incubation and rinse step. Sections were mounted on gelatin-coated slides, dried, dehydrated, and coverslipped. To avoid eventual bias, at least one animal from each group was included in every staining batch.
Cell Counting and Statistical Analysis
The nomenclature and nuclear boundaries defined in Swanson's stereotaxic rat brain atlas were used in this study (Swanson, 1992). c-Fos-immunoreactive (Fos-ir) nuclear profiles in different areas of the brain were counted using a Nikon microscope coupled to a video camera and a monitor. The boundaries of the brain areas were identified using adjacent Nissl-stained sections. A template or outline was developed for each brain nucleus or subnucleus based on the shape and the size of the region (Kollack-Walker et al, 1997). The location and relative size of each template are illustrated in Figure 2. The number of c-Fos-positive cell nuclei within each area was counted bilaterally (where possible) in four to six consecutive sections per animal and the average of them was expressed as number of cells Fos-ir/10 000 μm2. For some of the evaluated nuclei, we decided to provide a single cell count (rather than evaluating each individual subnucleus) due to difficulties in consistently defining its internal boundaries (eg bed nuclei stria terminalis, ventral groups (BST‘v’) and bed nuclei stria terminalis, dorsal groups (BNST‘d’)) and ventrolateral medulla (VLM)). Stereological methods were not employed in this study due to potential bias associated with counts generated in this manner, such as uncertainties as to the extent to which antiserum penetrates through the thickness of the tissue sections and difficulties in defining the boundaries of the several cell groups of interest. Moreover, our interest was to make only relative comparisons of the strength of Fos induction as a function of the treatment status (Li and Sawchenko, 1998; Medeiros et al, 2003).
Diagram illustrating the templates and relative sizes of the different brain areas subjected to counting of Fos-immunoreactive cells. The levels were based on Swanson's Stereotaxic Atlas of the Rat Brain. For abbreviations see Table 1 legend.
Statistical analysis of c-Fos expression was performed by one-way ANOVA followed by Dunnett's post hoc test. The level of significance was set at P⩽0.05. All values are expressed as mean±SEM.
RESULTS
Staining of each individual Fos-ir cell varied from very intense (black) to very light (light brown). To avoid double counting or overcounting, only cells stained in dark brown or black were considered for counting purposes. Staining of the evaluated structures usually was seen over a wide range of intensity for the different cells on a given brain structure. In this sense, a decrease in the number of countable cells in a given brain structure was generally accompanied by an overall decrease in the intensity of staining in that structure (ie there seemed to be a reduction of light- and moderate-stained cells as well). In general, dark-stained cells did not tend to cluster but rather seemed to be randomly distributed on a given brain structure. Finally, animals with a strong reduction of staining in one given nucleus did not necessarily have reductions of similar degree in other nuclei, that is, we found some variation from animal to animal even for the same experimental group.
The c-Fos expression seen in the control group (animals submitted to saline and restraint) was similar to that previously reported for stress-induced c-Fos expression (Senba et al, 1993; Chen and Herbert, 1995). It was widely distributed in the brain, with moderate to intense staining in areas involved with the stress response, such as limbic structures and cell groups involved in neuroendocrine and autonomic control (Table 1). Reliable induction of Fos-ir was seen in the cortex, mainly in anterior cingulate area, primary motor area, and visceral cortex (not counted). Labeling in the other cortical areas, such as the hippocampus for example, was quite sparse, even though we consistently counted a few Fos-ir cells in the hippocampus area in each section. In the bed nucleus, labeling was very prominent and concentrated in the ventral nuclei (here grouped as BST‘v’). However, we also detected a robust c-Fos expression in the dorsal groups of bed nuclei (BST‘d’). Fos expression in the amygdaloid complex was generally moderate, and while all recognized subdivisions contained at least a few positive cells, the main part of these were distinctly concentrated in the central nucleus of amygdala (CEA). Fos induction in the septal complex comprised principally a distinctive wedge-shaped group of cells in the ventral part of the lateral septal nucleus (LSv) (Figure 4). Labeling in the intermediate and dorsal parts of the lateral septal nucleus was moderate and essentially absent in the medial septal nucleus. The most marked Fos induction in the thalamus was seen throughout many of the midline (paraventricular nucleus of the thalamus (PVT)) and intralaminar nuclei (central medial nucleus of the thalamus, CM), while Fos induction in other thalamic areas was not considerable. Restraint-induced Fos-ir in the hypothalamus was concentrated mainly in the PVH, suprachiasmatic (SCH) and supramammillary (SUMI), and preoptic and periventricular (PVp (posterior periventricular nucleus of the hypothalamus) and PVa (anterior periventricular nucleus of the hypothalamus)) nuclei. Fos-ir neurons in the PVH concentrated predominantly in the dorsal zone of medial parvocellular part, with moderate expression in almost all other parts of the parvocellular division, while scarce in the magnocellular zone (Figure 3). Like most acute systemic challenges, restraint also provoked Fos expression in several structures of brain stem, recognized as comprising a core of interconnected cell groups involved in central autonomic and neuroendocrine control, as LC, nucleus of tract solitary (NTS), parabrachial nucleus (PB), and VLM. In the PB, labeling was generally sparse and concentrated mainly in dorsal lateral subnuclei, while in the NTS, responsive neurons were concentrated mainly in the caudal two-thirds of its medial division. In the LC (Figures 4 and 5), labeling was robust and distributed in all of its extension. Restraint-sensitive neurons were distributed throughout the ventrolateral medullary reticular formation from the caudal pole of the facial motor nucleus to the spinal-medullary transition area (Li and Sawchenko, 1998). Similar patterns of c-Fos expression were previously described for footshock-induced c-Fos expression (Li and Sawchenko, 1998).
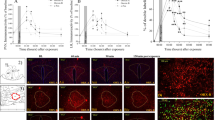
Photomicrograph illustrating the effect of dexamethasone, diazepam, and imipramine on stress-induced Fos-ir on the septal area. Animals were submitted to daily injections of saline (a–c) or imipramine (d) for 20 days. On the 21st day, animals received (a) saline, (b) dexamethasone (1 mg/kg), (c) diazepam (5 mg/kg), or (d) imipramine (15 mg/kg) before the restraint. After 1 h of restraint, the animals were evaluated for Fos-ir. Abbreviations: LV, lateral ventricle; MS, medial septum; LSv, lateral septal nucleus, ventral part; scale bar 140 μm.
Photomicrograph illustrating the effect of dexamethasone, diazepam, and imipramine on stress-induced Fos-ir on the PVH. Animals were submitted to daily injection of saline (a–c) or imipramine (d) for 20 days. On the 21st day, animals received (a) saline, (b) dexamethasone (1 mg/kg), (c) diazepam (5 mg/kg), or (d) imipramine (15 mg/kg) before the restraint. After 1 h of restraint, the animals were evaluated for Fos-ir. Abbreviations: V3, 3th ventricle; PVHmpd, paraventricular nucleus hypothalamus, medial parvicellular part, dorsal zone; pml, posterior magnocellular, lateral zone; scale bar 100 μm.
Photomicrograph illustrating the effect of dexamethasone, diazepam, and imipramine on stress-induced Fos-ir on the LC. Animals were submitted to daily injections of saline (a–c) or imipramine (d) for 20 days. On the 21st day, animals received (a) saline, (b) dexamethasone (1 mg/kg), (c) diazepam (5 mg/kg), or (d) imipramine (15 mg/kg) before the restraint. After 1 h of restraint, the animals were evaluated for Fos-ir. Abbreviation: V4, 4th ventricle; scale bar 150 μm.
Rats pretreated with dexamethasone and subsequently immobilized had significantly fewer Fos-ir cells in the SS cortex (60% reduction, SS=0.327±0.079 vs SDX=0.133±0.033), in the hippocampus, with 58–62% reduction in the fields CA1, CA2, and CA3, PVH (64% reduction), and LC (61% of reduction), as compared to animals pretreated with saline (Table 1 and Figures 3 and 5). Despite the lack of statistically significant difference, dexamethasone treatment also reduced by more than 50% the number of Fos-ir cells in the CEA, lateral habenula (LH), CM, SCH, medial preoptic area (MPO), PVp, and medial part of the NTS (Table 1).
Treatment with diazepam, as compared to saline controls, significantly decreased stress-induced c-Fos expression, yielding a 75% reduction in the SS cortex, between 57 and 64% reduction in different portions of the hippocampus (eg CA1, CA2, and CA3), 68% reduction in the bed nucleus of the stria terminalis, 66 and 80% reduction in the intermediate parts of the lateral septal nucleus and LSv, respectively (Figure 4), 56% in the MPO, and 43% in the SUMI. In other areas, despite the lack of statistically significant differences, diazepam caused a notable increase in Fos expression in the NTS (107%) as well as a marked reduction (72%) in the pallidus raphe nucleus.
Chronic administration of imipramine, as compared to saline controls, produced a marked reduction on the number of Fos-ir in the hippocampus (CA2 and CA3, with 48–52% fewer stained cells), in the PVH (51% less) and LC (46% less), and increased in the raphe pallidus nuclei (111% raise) (Table 1).
DISCUSSION
These results clearly show that dexamethasone, diazepam, and imipramine differentially modulate stress-induced c-Fos expression. The comparative analysis of the effects of these drugs on the modulation of brain activation can expand the comprehension of the mechanisms involved on the effects of these drugs on stress responses.
Dexamethasone
Our results have shown that dexamethasone reduced the c-Fos expression in areas related to sensory coding, such as the SS cortex, as well as in limbic structures (eg hippocampus), hypothalamic areas (PVH), and in areas related to autonomic and neuroendocrine control (eg LC).
Previous findings by Imaki and colleagues have shown that the chronic administration of dexamethasone inhibits the induction of c-fos mRNA and CRF mRNA in the PVH and plasma corticosterone and ACTH after stress (Imaki et al, 1995a, 1995b and 1995c).
Possible mechanisms involved
The effect of dexamethasone on stress-induced c-Fos expression can be explained by a downregulation of CRF through the inhibition of the effects of protein kinase A pathways on CRF gene expression at the transcription level (Imaki et al, 1995c). CRF induces a pattern of c-Fos expression similar to that induced by stress, and has been suggested to act in the CNS influencing many responses to stress, raising the possibility that a central CRF system may integrate multimodal components of the organismic response to stress (Smagin et al, 2001). Based on the close link between stress-induced c-Fos expression and mRNA of CRF, the effect of dexamethasone on the stress-induced c-Fos expression would be mainly in areas with high densities of CRF receptors. Our results are, at least in part, in agreement with this hypothesis, since all brain areas where we detected an effect of dexamethasone have a moderate to high density of mRNA or CRF receptors. However, a significant number of areas with a high density of CRF receptors, such as lateral septum, bed nucleus of terminalis stria, and dorsomedial nucleus of the hypothalamus (Smagin et al, 2001), did not present an altered Fos expression.
More recently, Ginsberg and colleagues have shown dissociation between c-fos and CRF gene expression. In this study, acute glucocorticoid treatment suppressed the secretion of ACTH and CRF gene expression, but did not affect c-Fos expression in the PVH (Ginsberg et al, 2003). Irrespective of the mechanism involved in triggering c-Fos expression in this area, which was not the subject of the current investigation, there was a clear effect of dexamethasone in the stress-induced c-Fos expression in the PVH.
In addition, the effect of dexamethasone in different brain structures could be discussed with regard to the distribution of glucocorticoid receptors. The inhibition on c-Fos expression caused by glucocorticoids has been related to an interaction between proteins of the AP-1 complex and the glucocorticoid–receptor complex. Binding of activated glucocorticoid receptor to proteins of AP-1 complex (Fos or c-Jun) can decrease the AP-1 activity and thus consequently c-Fos expression (Unlap and Jope, 1994). However, the areas in which we detected an effect of dexamethasone could not be directly related to the areas with a high density of glucocorticoid receptors (mainly type II that are more sensitive to dexamethasone). Here again, we have not detected a significant effect of dexamethasone on the stress-induced c-Fos expression on the lateral septum that has a high density of type II receptors (Magariños et al, 1989). On the other hand, we observed an effect of dexamethasone on the LC that has high reactivity to glucocorticoid receptors (Härfstrand et al, 1986).
Therefore, explanations based on CRF mechanisms or on the density of glucocorticoid receptors are not by themselves sufficient to explain the effects of dexamethasone on the restraint-induced c-Fos expression. The expression of c-Fos as a consequence of stress is the result of a complex interaction of pathways of cellular activation, and the effect of dexamethasone depends on the neurochemical identity of different cell type groups (eg, monoaminergic), the nature of the challenge (kind of stress), and the dose of corticosteroid among other factors (Li and Sawchenko, 1998; Ginsberg et al, 2003). Furthermore, not all corticosteroid-sensitive cells groups respond similarly to the manipulation of the levels of glucocorticoids (Li and Sawchenko, 1998).
Modulation of inputs to PVH
The stress-responsive CRF neurons of the PVH summate excitatory and inhibitory inputs into net secretory signals at the pituitary gland (Herman et al, 2003). Based on this view, dexamethasone reduced the activation not only of the PVH per se but also of the structures that send direct excitatory inputs to the PVH such as the LC (Ziegler et al, 1999). Besides, dexamethasone has also been shown to reduce the c-Fos expression of the hippocampus, another structure that modulates stress responses (Kovács et al, 1986). The hippocampal inhibition of the HPA is mediated by a set of neurons of the ventral subiculum that project extensively to BST and hypothalamic neurons relaying information to the PVH (Herman et al, 1995). Different from its effects on the LC, the effect of dexamethasone on the hippocampus cannot be directly related to a reduction of the activation of the PVH, given that reductions of the hippocampal activation are related to activation of the HPA axis. On the other hand, it has been suggested that the inhibition of the HPA axis represents a minor subset of hippocampal function and the steroids receptors, which are richly expressed throughout the hippocampus, can have other physiological actions, such as encoding the significance of stressful events into memory consolidation (Herman et al, 2003). These results suggest that dexamethasone has not only a direct effect on the PVH but also in structures that send excitatory inputs to PVH and in structures related to the coding of emotional stimulus.
Diazepam
Our results have shown that the treatment with diazepam also reduced the restraint-induced c-Fos expression in areas related to sensorial coding (eg SS cortex), in limbic structures (eg hippocampus, the bed nucleus of the stria terminalis, and the lateral septal nucleus), and in hypothalamic areas (eg MPO and the SUMI).
Previous results of Beck and Fibiger (1995) have shown that diazepam produced dose-related decreases in the conditioned stress-induced c-fos expression. Accordingly, the dose of 5 mg/kg of diazepam, but not 2.5 mg/kg, reduced Fos induction in the cingulate, piriform, infralimbic and retrosplenial cortices, CEA, LH, pretectal area, SUMI, and median and pontine of raphe. However, at 5 mg/kg these authors did not observe an effect in the LSv, PVT, CM, LC, and dorsal raphe nucleus (DR) (Beck and Fibiger, 1995). Our results agree, at least in part, with the above reported study. Both studies have detected an effect of diazepam in the SUMI, and not in the PVT, CM, LC, and DR. On the other hand, the results were distinct for a number of other studied areas. One possible explanation for these discrepant results is that different kinds of stress (eg conditioned fear vs restraint) can induce different patterns of c-Fos expression and, consequently, show distinct sensibility to diazepam. In this sense, administration of 10 mg/kg of diazepam provoked a clear reduction on the c-Fos expression induced by conditioned fear, whereas it did not have effect on the expression induced by nicotine (Beck and Fibiger, 1995; Salminen et al, 1996).
Possible mechanisms involved
One possible explanation for the specificity of the effect of diazepam in the reduction of c-Fos expression is the distribution of benzodiazepines receptors in the CNS. Areas showing high densities include the cerebral cortex, molecular layer of the cerebellum, and parts of the limbic system, including amygdaloid complex, hippocampal formation, piriform cortex, medial septal nucleus, hypothalamus, and olfactory bulb (Young and Kuhar, 1980; Richards and Möhler, 1984). We detected an important effect of diazepam in the lateral septal nucleus, which has a moderate density of receptors, and did not detect an effect on the amygdala, which has a high density of receptors. Therefore, similar to the results seen with dexamethasone, the areas where we detected an effect of diazepam cannot be directly related to the areas with a high density of related receptors, suggesting that density of BDZ receptors does not seem a critical factor for the encountered effects on the c-Fos expression induced by stress.
Modulation of inputs to PVH
Similar to what was discussed for dexamethasone, irrespective to the mechanisms, diazepam can change the stress responses by modulation of inhibitory and excitatory inputs to the HPA axis. Based on this view, although diazepam did not have any effect on the c-Fos expression on the PVH, it has reduced the activation of the MPO that sends direct excitatory projections to PVH (Cullinan et al, 1996). On the other hand, diazepam reduced the c-Fos expression in areas that can inhibit as well as activate the HPA axis. In this way, diazepam reduced c-Fos expression on the lateral septal area, which innervates PVH-projecting regions containing both GABAergic and glutamatergic neurons (Herman et al, 2003). Diazepam also reduced c-Fos expression in the hippocampus (Herman et al, 1995) and bed nuclei (anterolateral nuclei) (Casada and Dafny, 1991), areas that modulate the PVH. Diazepam also reduced c-Fos expression on SUMIs, which does not have a defined role on the excitability of the HPA. Although the mammillary nuclei receives considerable input from limbic forebrain structures and sends dense innervation to PVH (Price, 1995), the function of these regions in the stress responses is ill defined (Herman et al, 2003). Analysis of these data suggests that the effect of diazepam directly on the PVH or indirectly on the excitatory and inhibitory inputs to the HPA was not sufficient to reduce the activation of PVH. In spite of this, the brain areas where we detected an effect of diazepam could be related to other aspects of the stress response. The lateral septum, for example, has been suggested to be a key mediator of anxiety states and the principal site of action for antianxiety drugs (Gray and McNaughton, 2000). In this sense, the diazepam effect could be more related to the encoding of stressful events and not only in the modulation of neuroendocrine responses to stress.
Imipramine
Chronic treatment with 15 mg/kg daily of imipramine reduced the c-Fos expression induced by restraint in limbic structures (eg hippocampus), in areas related to autonomic and neuroendocrine control (eg PVH and LC), and increased it in brainstem structures (eg raphe pallidus nuclei).
Previous results have shown that chronic, but not acute treatment with imipramine (Morinobu et al, 1995), reduced the swim stress-induced c-Fos expression in the PVH and in limbic cortical regions, including the medial prefrontal, ventrolateral orbital, and cingulate cortices, while no effect was detected in the septal area, LH, amygdaloid complex, and PVT (Duncan et al, 1996). Our results are in agreement with those of Duncan and colleagues for some of the seemingly more important structures studied, such as PVH, amygdaloid complex, habenula, and the lateral septal area. For the cingulate cortex and the bed nucleus, however, our results are opposite to that reported above (Duncan et al, 1996).
Possible mechanisms involved
The mechanism(s) that underlie the downregulation of c-fos induction by antidepressants could involve long-term adaptations of neuronal function in response to chronic treatment (Morinobu et al, 1995). It has been suggested that the downregulation of β-adrenergic receptors and of some subtypes of serotonin receptors (Heninger and Charney, 1987), adaptations in intracellular mechanisms, such as the cAMP levels (Duman et al, 1994), and activation of autoregulatory mechanisms (Morinobu et al, 1995) can contribute to the antidepressant effect on the c-Fos expression induced by stress. However, none of these can separately explain this effect.
Modulation of inputs to PVH
The effect of antidepressants on the c-Fos induction in the PVH has been previously reported for different kinds of stressors (Duncan et al, 1996) and is consistent with the clinical effects of antidepressants. Antidepressant administration normalizes HPA hyperactivity that is frequently observed in depressed patients and this effect is related to remission of depressive symptoms (Linkowski et al, 1987). This reduction of the HPA axis is, at least in part, consistent with our results showing a reduction in the c-Fos expression in the PVH and in the LC that send direct excitatory projections to PVH (Duman et al, 1994). The chronic administration of antidepressant reduced the burst rate and the tyrosine hydroxylase expression of LC (Nestler et al, 1990). The effect of stress on the LC is mediated by CRF, which stimulates the adenylcyclase and the cAMP levels in the LC (Duman et al, 1994). It suggests that the reduction caused by imipramine, at least in the LC, would balance the action of CRF and, consequently, reduce c-Fos expression. On the other hand, imipramine reduced the c-Fos expression in areas that send inhibitory projections to PHV, such as hippocampal CA2, and increased in the raphe pallidus nuclei that have unidentified functions in the HPA integration (Herman et al, 2003).
Overview
Comparing the effects of the studied drugs, differently from dexamethasone and imipramine, diazepam did not affect areas directly related to neuroendocrine output, such as the PVH, or areas related to autonomic output, such as the LC. In addition, the changes in other brain areas were also not uniform, with diazepam not significantly affecting Fos expression in the amygdala and imipramine increasing the activation in the PR.
Hierarchical circuitry controlling HPA responsiveness
Based on the view of Herman and colleagues, the central mechanisms of stress integration would be the result of an hierarchical circuitry controlling HPA responsiveness, where the PVH summates excitatory and inhibitory inputs into a net secretory signal at the pituitary gland (Herman et al, 2003). Some structures that innervate PVH neurons are primed to relay sensory information and promote responses to emergent homeostatic challenges, while other limbic structures are capable of activating the PVH in the absence of frank physiological challenges responding in an ‘anticipatory’ manner. According to this view, drugs that act in the stress response should promote early changes of the HPA responsiveness by direct action in PVH neurons or indirectly by modulation of inputs, mainly inhibiting excitatory or stimulating inhibitory projections. In this sense, dexamethasone and imipramine were able to reduce the c-Fos expression in the PVH and some of the regions that send excitatory inputs to PVH. However, these drugs also reduced c-Fos expression in structures that send inhibitory inputs to PVH; furthermore, imipramine also increased the c-Fos expression in some regions that do not have a defined function in the HPA integration, even though it sends excitatory inputs to PVH. Therefore, despite the evident limitations of this circuit model, our hypothesis is that dexamethasone and imipramine can reduce the stress responses by modulation of both a lower circuitry responsible by primary responses to stress (reflexive pathway) and a higher circuitry related to the HPA responsiveness and to encoding of stressful events (anticipatory pathway). On the other hand, diazepam did neither affect the PVH and nor reduced significantly c-Fos expression of structures directly related to the HPA axis excitability. Given the well-established clinical and experimental effects of diazepam on stress responses, it seems likely that its effects occur by modulation of neuronal areas mainly related to encoding the significance of a stressful event (anticipatory pathway).
Initiation and cessation of stress responses appears to be choreographed by sets of hierarchical circuits converting mono- or poly-sensory information into an integrated PVH response. However, it is very hard to predict the significance of each circuit on the stress response. A number of inputs to the PVH are peptidergic or do not yet have a clearly defined neurotransmitter, thus confusing the understanding of the precise action of each input on HPA responsiveness. Furthermore, in a broader level, the kind of stress represents an important experimental variable, which should be considered, given that the magnitude of many stress responses, including c-Fos expression, is stimulus specific.
Comparing with the effect of the repeated stress, the effect of these drugs on the stress-induced c-Fos expression was restricted to some brain structures. Immobilization (2 h) for 13 consecutive days was able to reduce the immobilization-induced c-Fos expression in most brain structures, including amygdala, hypothalamus, thalamic nuclei, and cortical areas (Medeiros et al, 2003). In this sense, the wide effect of repeated stress can be related to the fact that the habituation acted simultaneously in many circuits independently of which neurotransmitters or intracellular mechanisms were involved. In addition, even considering the potential interaction between different neurotransmitting systems and the action of a single drug in different intracellular mechanisms, the effect of pharmacological agents are likely to be more restricted.
The study of the consequences of different drugs on the c-Fos expression induced by stress can help in the understanding of the clinical effects of these drugs. The neuronal activation induced by different kinds of stress has distinct sensitivity to the administration of anxiolitics (Bozas et al, 1997). Antidepressants also act differently in the reduction of stress-induced c-Fos expression, and this antagonism has been used for a functional classification of antidepressants (Duncan et al, 1996). Recently, Sumner and colleagues have concluded, in a meta-analysis study, that differences between drugs classes support their classification by means of c-fos profiling and that differences within classes may reflect mechanistic variations (Sumner et al, 2004).
Despite the large number of studies evaluating the effects of different clinically relevant drugs on several different models of stress, there is relatively few comparative studies using the same model with different drugs. Furthermore, when available, these studies most often assess the effects of different agents only in a restricted number of brain structures. Our current effort in evaluating three different prototypic compounds in a vast number of brain structures thus provides an important comparative background that may help in the further understanding of the areas involved in stress and the effects of these compounds on stress.
References
Beck CHM, Fibiger HC (1995). Conditional fear-induced changes in behavior and in the expression of the immediate early gene c-fos: with and without diazepam pretreatment. J Neurosci 15: 709–720.
Bozas E, Tritos N, Phillipidis H, Stylianopoulou F (1997). At least three neurotransmitter system mediated a stress-induced increased in c-fos mRNA in different rat brain areas. Cell Mol Neurobiol 17: 157–169.
Casada J, Dafny N (1991). Restraint and stimulation of the bed nucleus of the stria terminalis produce similar stress-like behaviors. Brain Res Bull 27: 207–212.
Chen X, Herbert J (1995). Regional changes on c-fos expression in the basal forebrain and brainstem during adaptation to repeated stress: correlations with cardiovascular, hypothermic and endocrine responses. Neuroscience 64: 675–685.
Cullinan WE, Helmreich DL, Watson SJ (1996). Fos expression in forebrain afferents to the hypothalamic paraventricular nucleus following swim stress. J Comp Neurol 368: 88–99.
Cullinan WE, Herman JP, Battaglia DF, Akil H, Watson SJ (1995). Pattern and time course of immediate early gene expression in rat brain following acute stress. Neuroscience 64: 477–505.
Duman R, Heninger GR, Nestler EJ (1994). Adaptations of receptor-coupled signal transduction pathways underlying stress- and drug-induced neural plasticity. J Nerv Ment Dis 182: 692–700.
Duncan GE, Knapp DJ, Johnson KB, Breese GR (1996). Functional classification of antidepressant based on antagonism of swim stress-induced Fos-like immunoreactivity. J Pharmacol Exp Ther 277: 1076–1079.
Ginsberg AB, Campeau S, Day HE, Spencer RL (2003). Acute glucocorticoid pretreatment suppresses stress-induced hypothalamic-pituitary-adrenal axis hormone secretion and expression of corticotropin-releasing hormone hnRN but does not affect c-fos mRNA or Fos protein expression in the paraventricular nucleus of hypothalamus. J Neuroendocrinol 15: 1075–1083.
Gray JA, McNaughton N (2000). The Neuropsychology of Anxiety. Oxford University Press: Oxford.
Härfstrand A, Fuxe K, Cintra A, Agnati LF, Zini I, Wikström A-C et al (1986). Glucocorticoid receptor immunoreactivity in monoaminergic neurons of rat brain. Proc Natl Acad Sci USA 83: 9779–9783.
Heninger GR, Charney DS (1987). Mechanism of action of antidepressant treatments: implications for the etiology and treatment of depressive disorders. In: Meltzer H (ed). Psychopharmacology. Raven Press: New York. pp 535–544.
Herman JP, Cullinan WE, Morano MI, Akil H, Watson SJ (1995). Contribution of the ventral subiculum to inhibitory regulation of the hypothalamo-pituitary-adrenocortical axis. J Neuroendocrinol 7: 475–482.
Herman JP, Figueiredo H, Mueller NK, Ulrich-Lai Y, Ostrander MM, Choi DC et al (2003). Central mechanism of stress integration: hierarchical circuitry controlling hypothalamo-pituitary-adrenocortical responsiveness. Front Neuroendocrinol 24: 151–180.
Imaki T, Shibasaki T, Hotta M, Demura H (1995a). Intracerebroventricular administration of corticotropin-releasing factor induces c-fos mRNA expression in brain regions related to stress responses: comparison with pattern of c-fos mRNA induction after stress. Brain Res 616: 114–125.
Imaki T, Wang X-Q, Shibasaki T, Chikada N, Takahashi C, Naruse M (1995b). Chlordiazepoxide attenuates stress-induced activation of neurons, corticotropin-releasing factor (CRF) gene transcription and CRF biosynthesis in the paraventricular nucleus (PVN). Mol Brain Res 32: 261–270.
Imaki T, Xiao-Quan W, Shibasaki T, Yamada K, Harada S, Chikada N et al (1995c). Stress-induced activation of neuronal activity and corticotropin-releasing factor gene expression in the paraventricular nucleus is modulated by glucocorticoids in rats. J Clin Invest 96: 231–238.
Kollack-Walker S, Watson SJ, Akil H (1997). Social stress in hamsters: defeat activates specific neurocircuits within the brain. J Neurosci 17: 8842–8855.
Kovács KJ, Kiss JZ, Makara GB (1986). Glucocorticoid implants around the hypothalamic paraventricular nucleus prevent the increase of corticotropin-releasing factor and arginine vasopressin immunostaining induced by adrenalectomy. Neuroendocrinology 44: 229–234.
Lachuer J, Delton I, Buda M, Tappaz M (1994). The habituation of brainstem catecholaminergic groups to chronic daily restraint stress is stress specific like that of the hypothalamo-pituitary-adrenal axis. Brain Res 638: 196–202.
Linkowski P, Mendlewicz J, Kerkhofs M, Leclercq R, Golstein J, Brasseur M et al (1987). 24-hour profiles of adrenocorticotropin, cortisol, and growth hormone in major depressive illness: effect of antidepressant treatment. J Clin Endocrinol Metab 65: 141–152.
Li X-Y, Sawchenko PE (1998). Hypothalamic effector neurons and extended circuitries activated in ‘neurogenic’ stress: a comparison of footshock effects exerted acutely, chronically, and in animals with controlled glucocorticoid levels. J Comp Neurol 393: 244–266.
Magariños AM, Ferrini M, De Nicola AF (1989). Corticosteroid receptors and glucocorticoid content in microdissected brain regions: correlative aspects. Neuroendocrinology 50: 673–678.
Medeiros MA, Canteras N, Suchecki D, Mello LEAM (2003). c-Fos expression induced by electroacupuncture at the Zusanli point in rats submitted to repeatted immobilization. Braz J Biol Med Res 36: 1673–1684.
Morinobu S, Nibuya M, Duman R (1995). Chronic antidepressant treatment down-regulates the induction of c-fos mRNA in response to acute stress in rat frontal cortex. Neuropsychopharmacology 12: 221–228.
Nestler EJ, McMahon A, Sabban E, Tallman JF, Duman R (1990). Chronic antidepressant administration decreased the expression of tyrosine hydroxylase in the rat locus coeruleus. Proc Natl Acad Sci USA 87: 7522–7526.
Price JL (1995). Thalamus. In: Paxinos G (ed). The Rat Nervous System. Academic Press: Sidney. pp 629–648.
Richards JG, Möhler H (1984). Benzodiazepine receptors. Neuropharmacology 23: 233–242.
Salminen O, Lahtinen S, Ahtee L (1996). Expression of Fos protein in various rat brain areas following acute nicotine and diazepam. Pharmacol Biochem Behav 54: 241–248.
Senba E, Matsunaga K, Tohyama M, Nogushi K (1993). Stress-induced c-fos expression in the rat brain: activation mechanism of sympathetic pathway. Brain Res Bull 31: 329–344.
Smagin GN, Heinrichs SC, Dunn A (2001). The role of CRF in behavioral responses to stress. Peptides 22: 713–724.
Sumner BEH, Cruise LA, Slattery DA, Hill DR, Shahid M, Henry B (2004). Testing the validity of c-fos expression profiling to aid the therapeutic classification of psychoactive drugs. Psychopharmacology 171: 306–321.
Swanson LW (1992). Brain Maps: Structure of the Rat Brain. Elsevier: Amsterdam.
Unlap T, Jope R (1994). Dexamethasone attenuates kainate-induced AP-1 activation in rat brain. Mol Brain Res 24: 275–282.
Young WS, Kuhar MJ (1980). Radiohistochemical lacalization of benzodiazepine receptors in the rat brain. J Pharmacol Exp Ther 212: 337–346.
Ziegler DR, Cass WA, Herman JP (1999). Excitatory influence of he locus coeruleus in hypothalamic pituitary adrenocortical axis responses to stress. J Neuroendocrinol 11: 361–369.
Acknowledgements
This work was supported by FAPESP (99/06114-9), FAPESP-CEPID, and Institutos do Milênio-CNPq; MAM was an FAPESP fellow (98/15751-0). We thank Dr Deborah Suchecki for suggestions and critical reading of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
de Medeiros, M., Carlos Reis, L. & Eugênio Mello, L. Stress-Induced c-Fos Expression is Differentially Modulated by Dexamethasone, Diazepam and Imipramine. Neuropsychopharmacol 30, 1246–1256 (2005). https://doi.org/10.1038/sj.npp.1300694
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.npp.1300694
Keywords
This article is cited by
-
Central regulation of stress-evoked peripheral immune responses
Nature Reviews Neuroscience (2023)
-
Memory-enhancing properties of sleep depend on the oscillatory amplitude of norepinephrine
Nature Neuroscience (2022)
-
Selective activation of parvalbumin interneurons prevents stress-induced synapse loss and perceptual defects
Molecular Psychiatry (2018)
-
Comparison of chronic physical and emotional social defeat stress effects on mesocorticolimbic circuit activation and voluntary consumption of morphine
Scientific Reports (2017)
-
Examining face and construct validity of a noninvasive model of panic disorder in Lister-hooded rats
Psychopharmacology (2010)