Abstract
Brain monoaminergic function is involved in the pathophysiology of psychiatric disorders. The loudness dependence (LD) of the N1/P2 component of auditory evoked potentials has been proposed as a noninvasive indicator of central serotonergic function, whereas single photon emission computed tomography (SPECT) and [123I]β-CIT can be used to visualize both serotonin (SERT) and dopamine transporters (DAT). The aim of the study was to correlate LD and SPECT measures in patients with obsessive-compulsive disorder, a condition with evidence for a serotonergic dysfunction. A total of 10 subjects received both neurophysiological and imaging investigations. Evoked potentials were recorded following the application of acoustic stimuli with increasing intensities. The LD of the relevant subcomponents (tangential dipoles) was investigated using dipole source analysis. SPECT was performed 20–24 h after injection of a mean 140 MBq [123I]β-CIT. As a measure of brain SERT and DAT availabilities, a ratio of specific to nonspecific [123I]β-CIT binding for the midbrain·pons region (SERT) and the striatum (DAT) was used. The LD of the right tangential dipole correlated significantly with both SERT and DAT availabilities (Pearson's correlations: ρ=0.69, p<0.05, and ρ=0.80, p<0.01, respectively). The correlations remained significant after controlling for the effects of age, gender, and severity of clinical symptoms. Associations between LD and both SERT and DAT availabilities further validate the use of neurophysiological approaches as noninvasive indirect measures of neurochemical brain function and point at a hypothesized interconnection of central monoaminergic systems.
Similar content being viewed by others
INTRODUCTION
Central monoaminergic neurotransmission plays a major role in the pathophysiology of neuropsychiatric disorders. The in vivo assessment of serotonergic and/or dopaminergic neurotransmission is an important field of research, since valid indicators of neurochemical brain function could be useful for both diagnostics and treatment decisions.
Peripheral measures such as serum levels of monoamines or their metabolites only partially reflect neurochemical brain function (Murphy, 1990) and have not been established in clinical practice. A neurophysiological approach, the assessment of the loudness dependence (LD) of the auditory evoked N1/P2 component, has been shown to be modulated by changes in central serotonergic activity in animal experiments (Juckel et al, 1997, 1999) and was proposed as a valid noninvasive indicator of serotonergic function in humans (Hegerl and Juckel, 1993). The LD describes changes in the amplitudes of event-related auditory evoked potentials (AEPs) elicited by different stimulus intensities. Since the auditory evoked N1/P2 potential is generated by overlapping subcomponents, dipole source analysis (DSA) has been used to differentiate a tangentially oriented dipole, mainly representing the activity of the primary auditory cortex, which shows a high rate of serotonergic innervation (Lewis et al, 1986; Näätänen and Picton, 1987; Scherg et al, 1989; Scherg and Picton, 1991; Scherg and Berg, 1996; Hegerl et al, 1994). A high LD of the tangential dipole has been associated with a low function of serotonergic neurotransmission and vice versa. In clinical studies, an increased LD has been shown in MDMA users (Tuchtenhagen et al, 2000; Croft et al, 2001) and patients with borderline personality disorder (Norra et al, 2003), indicative of a serotonergic dysfunction in these subjects. In depressed patients, a high LD before drug treatment has been associated with a favorable response to serotonergic medication (Hegerl and Juckel, 1993; Gallinat et al, 2000; Mulert et al, 2002). The observation that allelic variants of the serotonin transporter gene differ with respect to the LD further supports the hypothesis of an association between the serotonergic system and neurophysiological measures (Gallinat et al, 2003). However, some earlier studies showed that a high-intensity dependence of auditory and visual evoked potentials was also related to low levels of dopamine metabolites in cerebrospinal fluid or urine (von Knorring and Perris, 1981; Bruneau et al, 1986), suggesting interactions between both brain serotonergic and dopaminergic activities.
The development of new radioligands for neuroimaging techniques has further facilitated the investigation of central neurotransmitter systems. [123I]-2β-carbomethoxy-3β-(4-iodophenyl)tropane ([123I]β-CIT) is a radiotracer with high affinity to monoamine transporters (Innis et al, 1991; Boja et al, 1992) and thus can be used to visualize the central serotonin (SERT) and dopamine transporters (DAT) of the human brain in vivo with single photon emission computed tomography (SPECT) (Innis et al, 1993; Kuikka et al, 1993; Brücke et al, 1993; Pirker et al, 2000). β-CIT has been shown to accumulate in distinct brain areas, primarily in striatal, diencephalic, midbrain, and brainstem regions (Innis et al, 1991; Laruelle et al, 1993). In the striatum, the density of DAT is much higher than that of SERT, and in vivo displacement studies in nonhuman primates have shown that striatal β-CIT uptake mainly reflects binding to DAT (Laruelle et al, 1993). In the other regions comprising hypothalamus, thalamus, midbrain, and pons, β-CIT uptake has been predominantly associated with SERT availability according to in vivo displacement studies and autoradiographic post-mortem studies (Laruelle et al, 1993; Staley et al, 1994; Pirker et al, 1995). The stable uptake of β-CIT in SERT- and DAT-rich brain regions between 20 and 24 h after injection suggests that both SERT and DAT availabilities can be assessed within the same session (Pirker et al, 2000). β-CIT–SPECT studies in psychiatric disorders with suspected serotonergic pathomechanism have shown a reduced serotonin transporter binding in patients with major depression, alcoholism, seasonal affective disorder, or bulimia (Malison et al, 1998; Heinz et al, 1998; Willeit et al, 2000; Tauscher et al, 2001), and an increased serotonin transporter binding in acutely abstinent cocaine-dependent patients and patients with obsessive-compulsive disorder (OCD) (Jacobsen et al, 2000; Pogarell et al, 2003).
The aim of the present study was to investigate and compare different measures of monoaminergic function and to correlate neurophysiological (LD) and imaging (SPECT and β-CIT) variables within the same group of patients. For this purpose, patients with OCD were investigated who received both AEP recordings and β-CIT–SPECT. OCD is a clinically defined condition, characterized by the occurrence of intrusive and inappropriate repetitive thoughts or behaviors with evidence of a pathophysiologically relevant serotonergic dysfunction (Leonard et al, 1988; Goodman, 1999; Stein, 2002). A recently published β-CIT–SPECT study in nine patients with OCD showed an increase in SERT but not in DAT availability as compared to healthy control subjects (Pogarell et al, 2003).
METHODS
The study was approved by the local ethics committee (University of Munich) and by federal regulatory authorities in terms of the use of radioactive agents. All subjects gave written informed consent for participation in this study, after the neurophysiological and imaging procedures had been fully explained by the research physicians of the departments of psychiatry and nuclear medicine.
Subjects
The study population consisted of 10 patients (five males) with OCD ranging in age from 21 to 54 years (mean±standard deviation (SD): 33.6±11.1 years). Eight of the subjects participated in a β-CIT–SPECT project that was designed to characterize patients with OCD vs matched healthy control subjects regarding SERT and DAT availability, reported elsewhere (Pogarell et al, 2003). The patients were recruited at the Department of Psychiatry, University of Munich, and the Psychosomatic Hospital Windach and were diagnosed by experienced psychiatrists fulfilling the criteria for OCD according to the Diagnostic and Statistical Manual of Mental Disorders (DSM IV; American Psychiatric Association, 1994). Exclusion criteria were an age below 20 or above 60 years, comorbid psychiatric disorders (DSM IV axis I or II), as assessed by structured interviews and checklists, adopted from the structured clinical interview for DSM IV (SCID, German version; Wittchen et al, 1997), or any other medical or neurological illnesses. Female subjects were required to have a negative urine pregnancy test, performed immediately before the application of the radiotracer. The patients had to be unmedicated regarding any psychotropic medication for at least 12 weeks. Three patients had a history of psychopharmacotherapy. Two had temporarily received tricyclic antidepressants (clomipramine, n=1; imipramine, n=1), and one patient was treated with paroxetine a couple of years ago. The drug treatments were stopped due to lack of efficacy several months to years before study inclusion. Seven of the 10 patients were drug naïve (never medicated), and all of the subjects were nonsmokers. Signs and symptoms of the disorder were clinically rated using the Yale–Brown Obsessive-Compulsive Scale (Y-BOCS; Goodman et al, 1989a, 1989b), and additional depressive symptoms were assessed with the Beck Depression Inventory (Beck et al, 1961). The rating scales were administered immediately prior to the SPECT imaging and yielded mean (±SD) scores of 22.3±7.7 (Y-BOCS, range 10–36) and 17.1±9.4 (BDI, range 4–29). Patients underwent both the SPECT and neurophysiological investigations within 5 days after study enrolment.
SPECT Imaging
The subjects were pretreated with perchlorate 30–60 min before intravenous injection of a mean 140 MBq [123I]β-CIT as a single bolus. SPECT scanning was performed 20–24 h after injection using a triple-headed gamma camera (Picker Prism 3000, Cleveland, OH) equipped with low-energy, high-resolution fan beam collimators. The acquisition parameters consisted of a rotational radius of 12.7–13 cm, a 20% energy window centered on 159 keV, 120 projection angles over 360° (60 s per projection), and a 128 × 128 matrix. Images were reconstructed by filtered backprojection (low-pass filter with a cutoff frequency of 0.27 Nyquist, fifth order) and corrected for attenuation according to Chang (1978) (μ=0.12 cm−1). Voxel size in the reconstructed and reoriented images was 2.26 × 2.26 × 2.26 mm.
Image Analysis
The analysis of the SPECT images was conducted in a blinded fashion regarding the subjects’ diagnoses or clinical states. For regions-of-interest (ROIs) analysis, transaxial slices were reformatted by drawing a line connecting the anterior-most aspect of the frontal pole to the posterior-most aspect of the occipital pole, which can be considered as an approximation of the AC–PC line (Mozley et al, 1996). In accordance with brain imaging guidelines (Tatsch et al, 2002) and other authors (Mozley et al, 1996; Pogarell et al, 2003), this approach allows a sufficiently standardized and reproducible plane reorientation.
For the assessment of serotonin transporter availability, six consecutive transaxial slices (slice thickness 2.26 mm) with the highest midbrain and pons uptake of [123I]β-CIT were summed to yield a single transaxial slice (midbrain·pons) of 13.6 mm thickness. For further evaluation, standardized ROI templates for midbrain·pons (single circular template, 55 pixels in size) and the occipital cortex (637 pixels in size) were positioned on the summed slice. In addition, specific [123I]β-CIT binding in the striatum as a measure of DAT was evaluated in the same session. For this purpose, a standardized ROI template comprising the (left and right) striatum was applied to the 12 consecutive transaxial slices (slice thickness 2.26 mm each) presenting with the highest striatal tracer accumulation.
Assuming that [123I]β-CIT achieves a state of equilibrium binding in the brain by 20–24 h p.i. (Pirker et al, 2000), the ratios of specific to nonspecific brain uptake (V3″-SERT=(midbrain·pons–occipital)/occipital and V3″-DAT=(striatum–occipital)/occipital) reflect a measure proportional to the respective binding potential (Laruelle et al, 1994; Malison et al, 1998). The V3″-SERT was given as a single ratio for the midbrain·pons region, and V3″-DAT binding was calculated for the mean of left and right striatal uptake (mean striatum) and for both sides separately (left and right striatum). These ratios were used for all statistical analyses.
Neurophysiological Assessments
Subjects were seated in a comfortable armchair in an electrically shielded and sound-attenuated room. They were instructed to avoid movements and blinking during the recording. Sinus tones 1000 Hz, 40 ms duration with 10 ms rise and fall time, and an interstimulus interval randomized between 1800 and 2200 ms of five intensities 60, 70, 80, 90, and 100 dB sound pressure level (SPL) were presented in a pseudorandomized order through earphones. Electroencephalographic (EEG) activity was recorded with 32 tin electrodes placed via electrocaps according to the international 10/20 system, with Cz as reference and Fpz as ground electrodes. Additional electrodes (above the left eye and at the left ocular canthis) were used to monitor ocular artifacts. Impedances were kept at 5 kΩ or below. EEG was filtered using a band pass of 0.16–70 Hz and digitized at a sample rate of 250 Hz with an epoch length of 800 ms (200 ms prestimulus baseline). A total of 500 sweeps (100 for each intensity) were evaluated. Epochs with excessive eye or body movements (±50 mV) in any of the 32 channels were automatically rejected, as well as the first five responses to each intensity, to reduce short-term habituation effects. At least 40 artifact-free sweeps in any of the intensities were required. For each subject, the remaining sweeps were averaged separately for the five intensity levels.
Dipole Source Analysis
DSA was performed with Brain Electrical Source Analysis (BESA; Scherg and Picton, 1991), assuming that the cortical activity in the time range of the N1/P2 component would be adequately represented by the activity of two dipoles per hemisphere: a tangential and a radial dipole. The tangential dipole explains most of the variance of cortical activity at this latency range, shows the strongest LD, and mainly represents the activity of the primary auditory cortex, whereas the radial dipole represents the secondary auditory cortex activity (Hegerl et al, 1994). For analysis, the averaged curves of each subject were entered into BESA. A grand mean of the five averaged curves was calculated and used to adjust the dipole model individually. The individual curves were entered separately for the five intensities. The N1/P2 epoch amplitude or dipole moment, that is, the root-mean-squared effective amplitude over the epoch of the N1/P2 component (μVeff), was determined for the 66.7–233 ms epoch and obtained for each dipole (right and left sides) and intensity. LD was operationalized as an amplitude/intensity function slope on the basis of N1/P2 epoch amplitudes and calculated for the tangential and radial dipoles. A median slope in μVeff/10 dB was calculated from the slopes of all possible connections (n=10) between the five amplitude values to the five intensities (for details of the procedure, see Hegerl et al, 1994).
Statistical Analysis
Statistical analyses were performed using the Statistical Package for the Social Sciences (SPSS version 9.0.1 for Microsoft Windows). Descriptive analyses of clinical, neurophysiological, and SPECT variables are given as mean and SD. To explore the possible relationships between LD and the specific to nonspecific [123I]β-CIT binding ratios (midbrain·pons for SERT and striatum for DAT), Pearson's correlation coefficients (ρ) between β-CIT ratios and the LD of the dipoles of right and left hemispheres were calculated. To control the data for the effects of age, gender, or clinical variables, such as the severity of obsessive-compulsive (Y-BOCS scores) or depressive symptoms (BDI scores), a linear regression model was used with age, gender, and symptom scores as independent variables. The p<0.05 level was considered to be statistically significant. Due to the exploratory character of the study and the small sample size, no alpha correction was performed.
RESULTS
The mean (±SD) specific to nonspecific β-CIT binding ratio for midbrain·pons was 2.17±0.39. The binding ratios for mean, left striatum, and right striatum were 5.56±0.77, 5.59±0.83, and 5.50±0.75, respectively. The mean LD values of the tangential dipoles were 0.20±0.17 μVeff/10 dB for the left and 0.25±0.15 μVeff/10 dB for the right hemisphere. The data for the individual subjects are presented in Table 1.
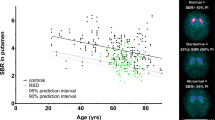
The LD of the right tangential dipole correlated significantly with both the midbrain·pons (Pearson's correlation, ρ=0.69, p<0.05) and mean striatal binding (Pearson's correlation, ρ=0.80, p<0.01) (Figures 1 and 2). Since LD turned out to be negatively correlated with age (Pearson's correlation, ρ=−0.64, p=0.05), a linear regression model was used to control for the effects of age, but also gender, and severity of obsessive-compulsive or depressive symptoms. The correlations between right LD and midbrain·pons and striatal binding remained significant after controlling the data for these effects (ρ=0.81, and 0.79, respectively, p<0.05).
LD of AEPs of the right side as assessed by DSA (tangential dipole of N1/P2 component) and specific striatal β-CIT binding (specific striatal to nonspecific occipital cortex binding ratio: striatum–occipital/occipital, mean of left and right sides) in 10 patients with OCD. Pearson's correlation: ρ=0.80, p<0.01.
Regarding DAT availability, both left and right striatal bindings correlated significantly with the right LD (ρ=0.74, p<0.05, and ρ=0.84, p<0.01, respectively).
No correlations were found for the LD of the left tangential dipoles and midbrain·pons or striatal binding (left tangential dipole and midbrain·pons: ρ=0.06, p=0.88; left tangential dipole and mean striatum: ρ=0.26, p=0.47).
Regarding the radial dipoles of the dipole source model, the mean LD values were 0.09±0.09 μVeff/10 dB (left hemisphere) and 0.11±0.13 μVeff/10 dB (right hemisphere). There were no significant correlations of the radial dipoles, neither with midbrain·pons (left radial dipole and midbrain·pons: ρ=−0.18, p=0.62; right radial dipole and midbrain·pons: ρ=0.13, p=0.73) nor striatal binding (left radial dipole and mean striatum: ρ=−0.08, p=0.82; right radial dipole and mean striatum: ρ=0.09, p=0.82).
DISCUSSION
A significant correlation was found between measures of two different tools for the assessment of monoaminergic function. SPECT imaging with the radioligand β-CIT allows one to assess semiquantitatively SERT and DAT availability by calculating binding ratios for the SERT-enriched midbrain·pons region and the striatum, where β-CIT binding is largely restricted to DAT (Innis et al, 1991; Laruelle et al, 1993; Staley et al, 1994). The LD of the auditory evoked N1/P2 component has been suggested to mainly reflect serotonergic properties of the auditory cortex. The implementation of DSA with a separation of two dipoles per hemisphere allows in part to focus on the brain activity of the primary auditory cortex, a brain region with a dense serotonergic innervation (Lewis et al, 1986; Scherg and Berg, 1996; Hegerl et al, 1994). However, up to date, there is no direct validation from human studies with independent measures that LD is modulated by brain serotonergic activity.
The clear positive correlation between neurophysiological and nuclear medicine measures in our study suggests that LD is related to central monoaminergic neurotransmitter systems as assessed by SPECT imaging. In our patients with OCD, the LD of the right primary auditory cortex (tangential dipole) was associated with both serotonin and dopamine transporter availabilities. Brain functional abnormalities in patients with OCD have repeatedly been shown in imaging and neurophysiological studies, and many of these investigations revealed more pronounced right-sided alterations (Baxter et al, 1992; Saxena et al, 1999; Saxena and Rauch, 2000). This could explain that in our study only the right LD, representing the right primary auditory cortex, revealed significant correlations with the imaging data.
Regarding the serotonergic system, a recently published β-CIT–SPECT study in patients with OCD as compared to healthy control subjects showed a significant increase in β-CIT binding to SERT-enriched regions in the patients, whereas striatal β-CIT binding did not differ between the groups (Pogarell et al, 2003). The elevated SERT availability in patients vs controls supports the hypothesis of a serotonergic dysfunction in OCD.
Likewise an increase in LD is suggestive of a serotonergic alteration in psychiatric patients, for example, in ecstasy users (Tuchtenhagen et al, 2000; Croft et al, 2001) or subjects with borderline personality disorder (Norra et al, 2003). Thus both a high LD and an elevated SERT availability are consistent with a disturbed serotonergic activity and this is in line with our observation of a positive correlation between these measures, that is, a high SERT status is associated with a high LD in patients with OCD and vice versa.
On the other hand, we found a clear correlation between the LD and striatal (DAT) β-CIT binding as well. This result was unexpected and revealed that there is no complete specificity of the LD for the serotonergic system. It has been shown that the intensity dependence of evoked potentials was associated with dopamine metabolites in CSF or urine in humans (von Knorring and Perris, 1981; Bruneau et al, 1986) and that the presence of the dopamine agonist apomorphine decreased the LD of the primary auditory cortex in cats (Juckel et al, 1997). These data point at dopaminergic influences on cortical processing of sensory stimuli. Due to the low dopaminergic innervation of sensory cortices (Lewis et al, 1987; Gaspar et al, 1989; Berger et al, 1991), these effects are supposed to be rather indirect (Juckel et al, 1997). Accordingly, in our study, only the tangential dipoles, representing the activity of a brain region with a dense serotonergic innervation, showed clear and significant correlations with the β-CIT binding and not the radial dipoles, which are associated with the activity of secondary auditory cortices without substantial serotonergic activity. This result again favors the serotonergic aspects of LD modulation. Nevertheless, the correlation of LD with dopaminergic measures, such as the striatal β-CIT binding (DAT availability), is consistent with the notion of central interconnections of the serotonergic and dopaminergic systems (Hervé et al, 1987; Parsons and Justice, 1993; Moukhles et al, 1997). Functional interactions between SERT and DAT have also been reported by Kugaya et al (2003) using β-CIT and SPECT in healthy subjects and patients with depression; the administration of antidepressant drugs (selective serotonin reuptake inhibitors (SSRIs)) in healthy controls and depressed patients led to a decrease in SERT and to an unexpected increase in DAT availability, suggesting an SSRI-induced modulation of the dopaminergic system. The patients in our study were free of psychotropic drugs; nevertheless, our data are suggestive of a link between the functional activity of the primary auditory cortex and both the serotonergic and the dopaminergic systems.
Some methodological limitations of this study need to be addressed. First, there was no MRI coregistration for the placement of ROIs and to identify brain regions with low activity (ie midbrain, pons) more accurately. However, to reduce variance, we have chosen standardized templates with fixed sizes for ROI delineation in accordance with studies by other groups (eg Malison et al, 1998; Tauscher et al, 2001).
Regarding the differentiation of SERT and DAT, there is a lack of selectivity of the radioligand β-CIT. Whereas β-CIT binding to the striatum, a well-defined region, is largely restricted to dopamine transporters (Innis et al, 1991; Laruelle et al, 1993), the SERT-enriched regions in diencephalon, midbrain, or brainstem are anatomically less circumscribed, and might be contaminated by other monoamine transporters, hampering the ROI analysis. Data from studies in nonhuman primates and post-mortem studies of the human brain showed that β-CIT could almost completely be displaced by SSRIs within the diencephalon and midbrain, indicating a predominant binding to SERT in these regions (Laruelle et al, 1993; Staley et al, 1994). Nevertheless, in vivo investigations of the human brain demonstrated that the reduction of β-CIT binding in these regions under various therapeutic doses of SSRIs was restricted to about 40–50% (Pirker et al, 1995; Tauscher et al, 1999; Kugaya et al, 2003), whereas comparable studies with more selective SERT ligands showed up to 100% occupancy of SERT under SSRI medication (Parsey et al, 2000; Meyer et al, 2001).
Besides the lack of selectivity of β-CIT, our approach to investigate DAT and SERT in the same SPECT session approximately 20–24 h after injection of the tracer is a matter of discussion and has been criticized. Ryding et al (2004) presented a new analytical model for the separation of DAT and SERT in β-CIT–SPECT measurements. Based on SPECT recordings at 1, 6, and 22 h after injection of β-CIT and using citalopram in order to block SERT uptake in the course of the experiment, the authors could demonstrate a successful separation of SERT and DAT, which is a clear advantage of this method compared with our approach. On the other hand, we have shown in an earlier study that only midbrain·pons and not striatal binding 20–24 h after injection of β-CIT was significantly different between patients with OCD and healthy subjects (Pogarell et al, 2003). This is again suggestive of functional differences between midbrain·pons and striatal monoamine transporters as assessed by SPECT and β-CIT 20–24 h after injection, and indicates that midbrain·pons binding is not completely contaminated, for example, by dopamine transporters.
Nevertheless, given methodological limitations, the SERT data of our study have to be interpreted with caution, since it cannot be excluded that β-CIT binding within the midbrain·pons region is affected by the presence of other monoamine transporters, especially DAT, and that the correlations reported here are largely restricted to dopamine transporter availability.
Thus it has to be taken into consideration that LD might be more clearly associated with SPECT variables of dopaminergic function, favoring dopaminergic rather than serotonergic aspects of LD. However, given functional interactions between monoaminergic systems (Moukhles et al, 1997; Kugaya et al, 2003), this would not be contradictory to the notion of LD as a clinical serotonergic marker (Hegerl et al, 2001; Croft et al, 2001). New models for β-CIT measurements with an improved separation of β-CIT binding to SERT and DAT as proposed by Ryding et al (2004), as well as the use of highly selective radioligands for SERT, such as ADAM for SPECT or DASB for PET (Oya et al, 2000; Kauppinen et al, 2003; Wilson et al, 2002; Frankle et al, 2004), might help to advance in vivo studies and to investigate the different aspects of central monoaminergic function more accurately.
To our knowledge, this is the first combined study of neurophysiological and SPECT imaging measures of monoaminergic function in patients with OCD. Our data suggest that LD might be a valid and useful measure of central neurochemical function, at least in patients with OCD, and further indicate a distinct inter-relation of serotonergic and dopaminergic mechanisms in the human brain.
References
American Psychiatric Association (1994). Diagnostic and Statistical Manual of Mental Disorders, 4th edn. American Psychiatric Press: Washington, DC.
Baxter Jr LR, Schwartz JM, Bergman KS, Szuba MP, Guze BH, Mazziotta JC et al (1992). Caudate glucose metabolic rate changes with both drug and behavior therapy for obsessive-compulsive disorder. Arch Gen Psychiatry 49: 681–689.
Beck AT, Ward CH, Mendelson J, Mock J, Erbaugh J (1961). An inventory for measuring depression. Arch Gen Psychiatry 4: 564–571.
Berger B, Gaspar P, Verney C (1991). Dopaminergic innervation of the cerebral cortex: unexpected differences between rodents and primates. Trends Neurosci 14: 21–27.
Boja JW, Mitchell WM, Patel A, Kopajtic TA, Carroll FI, Lewin AH et al (1992). High-affinity binding of [125I]RTI-55 to dopamine and serotonin transporters in rat brain. Synapse 12: 27–36.
Brücke T, Kornhuber J, Angelberger P, Asenbaum S, Frassine H, Podreka I (1993). SPECT imaging of dopamine and serotonin transporters with [123I]beta-CIT. Binding kinetics in the human brain. J Neural Transm Gen Sect 94: 137–146.
Bruneau N, Barthelemy C, Jouve J, Lelord G (1986). Frontal auditory-evoked potential augmenting-reducing and urinary homovanillic acid. Neuropsychobiology 16: 78–84.
Chang LT (1978). A method of attenuation correction in radionuclide computed tomography. IEEE Trans Nucl Sci 25: 638–643.
Croft RJ, Klugman A, Baldeweg T, Gruzelier JH (2001). Electrophysiological evidence of serotonergic impairment in long-term MDMA (‘ecstasy’) users. Am J Psychiatry 158: 1687–1692.
Frankle WG, Huang Y, Hwang DR, Talbot PS, Slifstein M, Van Heertum R et al (2004). Comparative evaluation of serotonin transporter radioligands 11C-DASB and 11C-McN 5652 in healthy humans. J Nucl Med 45: 682–694.
Gallinat J, Bottlender R, Juckel G, Munke-Puchner A, Stotz G, Kuss HJ et al (2000). The loudness dependency of the auditory evoked N1/P2-component as a predictor of the acute SSRI response in depression. Psychopharmacology 148: 404–411.
Gallinat J, Senkowski D, Wernicke C, Juckel G, Becker I, Sander T et al (2003). Allelic variants of the functional promoter polymorphism of the human serotonin transporter gene is associated with auditory cortical stimulus processing. Neuropsychopharmacology 28: 530–532.
Gaspar P, Berger B, Febvret A, Vigny A, Henry JP (1989). Catecholamine innervation of the human cerebral cortex as revealed by comparative immunohistochemistry of tyrosine hydroxylase and dopamine-beta-hydroxylase. J Comp Neurol 279: 249–271.
Goodman WK (1999). Obsessive-compulsive disorder: diagnosis and treatment. J Clin Psychiatry 60: 27–32.
Goodman WK, Price LH, Rasmussen SA, Mazure C, Delgado P, Heninger GR et al (1989a). The Yale–Brown Obsessive Compulsive Scale. II. Validity. Arch Gen Psychiatry 46: 1012–1016.
Goodman WK, Price LH, Rasmussen SA, Mazure C, Fleischmann RL, Hill CL et al (1989b). The Yale–Brown Obsessive Compulsive Scale. I. Development, use, and reliability. Arch Gen Psychiatry 46: 1006–1011.
Hegerl U, Gallinat J, Juckel G (2001). Event-related potentials. Do they reflect central serotonergic neurotransmission and do they predict clinical response to serotonin agonists? J Affect Disord 62: 93–100.
Hegerl U, Gallinat J, Mrowinski D (1994). Intensity dependence of auditory evoked dipole source activity. Int J Psychophysiol 17: 1–13.
Hegerl U, Juckel G (1993). Intensity dependence of auditory evoked potentials as an indicator of central serotonergic neurotransmission: a new hypothesis. Biol Psychiatry 33: 173–187.
Heinz A, Ragan P, Jones DW, Hommer D, Williams W, Knable MB et al (1998). Reduced central serotonin transporters in alcoholism. Am J Psychiatry 155: 1544–1549.
Hervé D, Pickel VM, Joh TH, Beaudet A (1987). Serotonin axon terminals in the ventral tegmental area of the rat: fine structure and synaptic input to dopaminergic neurons. Brain Res 435: 71–83.
Innis RB, Baldwin RM, Sybirska E, Zea Y, Laruelle M, Al-Tikriti M et al (1991). Single photon emission computed tomography imaging of monoamine reuptake sites in primate brain with [123I]CIT. Eur J Pharmacol 200: 369–370.
Innis RB, Seibyl JP, Scanley BE, Laruelle M, Abi-Dargham A, Wallace E et al (1993). Single photon emission computed tomographic imaging demonstrates loss of striatal dopamine transporters in Parkinson disease. Proc Natl Acad Sci USA 90: 11965–11969.
Jacobsen LK, Staley JK, Malison RT, Zoghbi SS, Seibyl JP, Kosten TR et al (2000). Elevated central serotonin transporter binding availability in acutely abstinent cocaine-dependent patients. Am J Psychiatry 157: 1134–1140.
Juckel G, Hegerl U, Molnar M, Csepe V, Karmos G (1999). Auditory evoked potentials reflect serotonergic neuronal activity—a study in behaving cats administered drugs acting on 5-HT1A autoreceptors in the dorsal raphe nucleus. Neuropsychopharmacology 21: 710–716.
Juckel G, Molnar M, Hegerl U, Csepe V, Karmos G (1997). Auditory-evoked potentials as indicator of brain serotonergic activity—first evidence in behaving cats. Biol Psychiatry 41: 1181–1195.
Kauppinen T, Bergstrom K, Heikman P, Hiltunen J, Ahonen A (2003). Biodistribution and radiation dosimetry of 123I ADAM in healthy subjects: preliminary results. Eur J Nucl Med Mol Imaging 30: 132–136.
Kugaya A, Seneca NM, Snyder PJ, Williams SA, Malison RT, Baldwin RM et al (2003). Changes in human in vivo serotonin and dopamine transporter availability during chronic antidepressant administration. Neuropsychopharmacology 28: 413–420.
Kuikka JT, Bergstrom KA, Vanninen E, Laulumaa V, Hartikainen P, Lansimies E (1993). Initial experience with single-photon emission tomography using iodine-123-labelled 2 beta-carbomethoxy-3 beta-(4-iodophenyl) tropane in human brain. Eur J Nucl Med 20: 783–786.
Laruelle M, Baldwin RM, Malison RT, Zea-Ponce Y, Zoghbi SS, Al-Tikriti MS et al (1993). SPECT imaging of dopamine and serotonin transporters with [123I]beta-CIT: pharmacological characterization of brain uptake in nonhuman primates. Synapse 13: 295–309.
Laruelle M, Wallace E, Seibyl JP, Baldwin RM, Zea-Ponce Y, Zoghbi SS et al (1994). Graphical, kinetic, and equilibrium analyses of in vivo [123I]beta-CIT binding to dopamine transporters in healthy human subjects. J Cereb Blood Flow Metab 14: 982–994.
Leonard H, Swedo S, Rapoport JL, Coffey M, Cheslow D (1988). Treatment of childhood obsessive compulsive disorder with clomipramine and desmethylimipramine: a double-blind crossover comparison. Psychopharmacol Bull 24: 93–95.
Lewis DA, Campbell MJ, Foote SL, Goldstein M, Morrison JH (1987). The distribution of tyrosine hydroxylase-immunoreactive fibers in primate neocortex is widespread but regionally specific. J Neurosci 7: 279–290.
Lewis DA, Campbell MJ, Foote SL, Morrison JH (1986). The monoaminergic innervation of primate neocortex. Hum Neurobiol 5: 181–188.
Malison RT, Price LH, Berman R, van Dyck CH, Pelton GH, Carpenter L et al (1998). Reduced brain serotonin transporter availability in major depression as measured by [123I]-2 beta-carbomethoxy-3 beta-(4-iodophenyl)tropane and single photon emission computed tomography. Biol Psychiatry 44: 1090–1098.
Meyer JH, Wilson AA, Ginovart N, Goulding V, Hussey D, Hood K et al (2001). Occupancy of serotonin transporters by paroxetine and citalopram during treatment of depression: a [(11)C]DASB PET imaging study. Am J Psychiatry 158: 1843–1849.
Moukhles H, Bosler O, Bolam JP, Vallee A, Umbriaco D, Geffard M et al (1997). Quantitative and morphometric data indicate precise cellular interactions between serotonin terminals and postsynaptic targets in rat substantia nigra. Neuroscience 76: 1159–1171.
Mozley PD, Kim HJ, Gur RC, Tatsch K, Muenz LR, McElgin WT et al (1996). Iodine-123-IPT SPECT imaging of CNS dopamine transporters: nonlinear effects of normal aging on striatal uptake values. J Nucl Med 37: 1965–1970.
Mulert C, Juckel G, Augustin H, Hegerl U (2002). A tomographic method for the loudness dependence analysis of the auditory evoked N1/P2-response in the primary auditory cortex. Clin Neurophysiol 113: 1566–1572.
Murphy DL (1990). Peripheral indices of central serotonin function in human. Ann NY Acad Sci 600: 282–296.
Näätänen R, Picton T (1987). The N1 wave of the human electric and magnetic response to sound: a review and an analysis of the component structure. Psychophysiology 24: 375–425.
Norra C, Mrazek M, Tuchtenhagen F, Gobbele R, Buchner H, Sass H et al (2003). Enhanced intensity dependence as a marker of low serotonergic neurotransmission in borderline personality disorder. J Psychiatr Res 37: 23–33.
Oya S, Choi SR, Hou C, Mu M, Kung MP, Acton PD et al (2000). 2-((2-((dimethylamino)methyl)phenyl)thio)-5-iodophenylamine (ADAM): an improved serotonin transporter ligand. Nucl Med Biol 27: 249–254.
Parsey RV, Kegeles LS, Hwang DR, Simpson N, Abi-Dargham A, Mawlawi O et al (2000). In vivo quantification of brain serotonin transporters in humans using [11C]McN 5652. J Nucl Med 41: 1465–1477.
Parsons LH, Justice Jr JB (1993). Perfusate serotonin increases extracellular dopamine in the nucleus accumbens as measured by in vivo microdialysis. Brain Res 606: 195–199.
Pirker W, Asenbaum S, Hauk M, Kandlhofer S, Tauscher J, Willeit M et al (2000). Imaging serotonin and dopamine transporters with 123I-beta-CIT SPECT: binding kinetics and effects of normal aging. J Nucl Med 41: 36–44.
Pirker W, Asenbaum S, Kasper S, Walter H, Angelberger P, Koch G et al (1995). Beta-CIT SPECT demonstrates blockade of 5HT-uptake sites by citalopram in the human brain in vivo. J Neural Transm Gen Sect 100: 247–256.
Pogarell O, Hamann C, Pöpperl G, Juckel G, Choukèr M, Zaudig M et al (2003). Elevated brain serotonin transporter availability in patients with obsessive-compulsive disorder. Biol Psychiatry 54: 1406–1413.
Ryding E, Lindstrom M, Bradvik B, Grabowski M, Bosson P, Traskman-Bendz L et al (2004). A new model for separation between brain dopamine and serotonin transporters in 123I-β-CIT SPECT measurements: normal values and sex and age dependence. Eur J Nucl Med Mol Imaging print copy in press (originally published online March 11, 2004, at http://springerlink.metapress.com/media/3D32QPRRVQCFD7NWDRWQ/Contributions/U/U/H/J/UUHJWMJPP1066495_html/fulltext.html).
Saxena S, Brody AL, Maidment KM, Dunkin JJ, Colgan M, Alborzian S et al (1999). Localized orbitofrontal and subcortical metabolic changes and predictors of response to paroxetine treatment in obsessive-compulsive disorder. Neuropsychopharmacology 21: 683–693.
Saxena S, Rauch SL (2000). Functional neuroimaging and the neuroanatomy of obsessive-compulsive disorder. Psychiatr Clin N Am 23: 563–586.
Scherg M, Berg P (1996). New concepts of brain source imaging and localization. Electroencephalogr Clin Neurophysiol Suppl 46: 127–137.
Scherg M, Picton TW (1991). Separation and identification of event-related potential components by brain electric source analysis. Electroencephalogr Clin Neurophysiol Suppl 42: 24–37.
Scherg M, Vajsar J, Picton TW (1989). Source analysis of the late human auditory evoked potentials. J Cogn Neurosci 4: 336–355.
Staley JK, Basile M, Flynn DD, Mash DC (1994). Visualizing dopamine and serotonin transporters in the human brain with the potent cocaine analogue [125I]RTI-55: in vitro binding and autoradiographic characterization. J Neurochem 62: 549–556.
Stein DJ (2002). Obsessive-compulsive disorder. Lancet 360: 397–405.
Tatsch K, Asenbaum S, Bartenstein P, Catafau A, Halldin C, Pilowski L et al (2002). EANM procedure guidelines for brain neurotransmission SPET using 123 I-labelled dopamine transporter ligands. Eur J Nucl Med Mol Imaging 29: BP30–BP35.
Tauscher J, Pirker W, de Zwaan M, Asenbaum S, Brucke T, Kasper S (1999). In vivo visualization of serotonin transporters in the human brain during fluoxetine treatment. Eur Neuropsychopharmacol 9: 177–179.
Tauscher J, Pirker W, Willeit M, de Zwaan M, Bailer U, Neumeister A et al (2001). [123I] beta-CIT and single photon emission computed tomography reveal reduced brain serotonin transporter availability in bulimia nervosa. Biol Psychiatry 49: 326–332.
Tuchtenhagen F, Daumann J, Norra C, Gobbele R, Becker S, Pelz S et al (2000). High intensity dependence of auditory evoked dipole source activity indicates decreased serotonergic activity in abstinent ecstasy (MDMA) users. Neuropsychopharmacology 22: 608–617.
Von Knorring L, Perris C (1981). Biochemistry of the augmenting-reducing response in visual evoked potentials. Neuropsychobiology 7: 1–8.
Willeit M, Praschak-Rieder N, Neumeister A, Pirker W, Asenbaum S, Vitouch O et al (2000). [123I]-beta-CIT SPECT imaging shows reduced brain serotonin transporter availability in drug-free depressed patients with seasonal affective disorder. Biol Psychiatry 47: 482–489.
Wilson AA, Ginovart N, Hussey D, Meyer J, Houle S (2002). In vitro and in vivo characterisation of [11C]-DASB: a probe for in vivo measurements of the serotonin transporter by positron emission tomography. Nucl Med Biol 29: 509–515.
Wittchen HU, Zaudig M, Fydrich T (1997). SKID—Strukturiertes Klinisches Interview Für DSM IV. Achse I and II: Handanweisungen. Hogrefe Verlag: Goettingen, Germany.
Acknowledgements
This work was supported by a grant from the Faculty of Medicine of the Ludwig-Maximilians-University of Munich (FöFoLe No. 119).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pogarell, O., Tatsch, K., Juckel, G. et al. Serotonin and Dopamine Transporter Availabilities Correlate with the Loudness Dependence of Auditory Evoked Potentials in Patients with Obsessive-Compulsive Disorder. Neuropsychopharmacol 29, 1910–1917 (2004). https://doi.org/10.1038/sj.npp.1300537
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.npp.1300537
Keywords
This article is cited by
-
Examination of the effect of acute levodopa administration on the loudness dependence of auditory evoked potentials (LDAEP) in humans
Psychopharmacology (2012)
-
Loudness dependence of auditory evoked potentials (LDAEP) in clinical monitoring of suicidal patients with major depression: a pilot study
European Archives of Psychiatry and Clinical Neuroscience (2012)
-
Chronic modulation of serotonergic neurotransmission with sertraline attenuates the loudness dependence of the auditory evoked potential in healthy participants
Psychopharmacology (2011)
-
Loudness dependence of auditory evoked potentials (LDAEP) correlates with the availability of dopamine transporters and serotonin transporters in healthy volunteers—a two isotopes SPECT study
Psychopharmacology (2011)
-
Fluvoxamine Treatment and D2 Receptors: a Pet Study on OCD Drug-Naïve Patients
Neuropsychopharmacology (2007)