Abstract
Corticotropin-releasing hormone (CRH) acts within the brain and pituitary to coordinate the overall endocrinological and behavioral stress response. From postnatal day (PND) 4 to 14, the infant rat displays minimal adrenal response to mild stress. However, maternal deprivation alters the pituitary–adrenal system such that the infants become responsive to specific stimuli. We hypothesized that maternal deprivation would also affect CRH brain circuits. Since tricyclic antidepressants have been shown to decrease the adrenal response to stress in adult rats, we hypothesized that CRH-related changes induced by maternal deprivation would be prevented by this treatment. Thus, we investigated CRH-related molecules on animals that were maternally deprived on PND 13 compared with nondeprived animals. We found that maternal deprivation caused alterations in the gene expression of both CRH receptors (CRHr) 1 and 2 in specific brain regions, and that some of these effects were augmented by chronic isotonic saline injections. There was a significant increase in CRH, CRHr1, and r2 mRNA in the cortex. In amygdala, CRHr1 and r2 mRNAs were decreased. CRHr2 mRNA was also decreased in the ventromedial nucleus of the hypothalamus, whereas an increase was detected in the hippocampal pyramidal cells. One week of desipramine (DES) administration preceding the maternal deprivation event prevented all the deprivation-induced changes in CRHr2 mRNA, regardless of the direction of the original change. We also found that chronic injection treatments enhanced the adrenocortical response and improved the efficiency of negative feedback in maternal deprivation animals. These results demonstrate that maternal deprivation elicits modifications of CRH brain circuits in a site-specific manner, and that the regulation of CRHr2 gene expression is mediated by mechanisms different from those involved with the modulation of CRHr1 in the infant rat.
Similar content being viewed by others
INTRODUCTION
The corticotropin-releasing hormone (CRH) brain system is viewed as a central coordinator of endocrinological, autonomic, and behavioral responses to stress (for a review see Herman and Cullinan, 1997). CRH neurons present in the paraventricular nucleus (PVN) of the hypothalamus are critical to the endocrine response to stress and constitute the final common path for the integration of the endocrinological response of the limbic–hypothalamic–pituitary–adrenal (LHPA) axis to stressful signals. CRH, along with a second secretagogue, arginine vasopressin (AVP), activates the anterior pituitary to release corticotropin (ACTH), which in turn interacts with the adrenal cortex causing elevation of stress steroids, corticosterone (CORT) in rodents, and cortisol in primates.
In addition to a neuroendocrine regulation role, CRH has a neuromodulatory role in the brain. Consistent with a neurotransmitter role, the CRH receptors (CRHr) 1 and 2 are expressed in diverse brain areas, including various cortical laminae, subcortical, limbic, and brainstem structures such as amygdala, bed nucleus of the stria terminalis, raphe nuclei, and locus ceruleus (Chalmers et al, 1996; Wong et al, 1994). CRHr1 appears to be expressed in the neocortex, cerebellar and sensory relay structures, and has a high affinity for the CRH peptide itself (Chalmers et al, 1996; Perrin and Vale, 1999). CRHr2 is expressed in more circumscribed brain regions, and recognizes both CRH and the urocortins (Vaughan et al, 1995). When administered centrally to adult rats, CRH elicits a wide spectrum of autonomic, electrophysiologic, and behavioral effects that appear to integrate perception and expression of fear or anxiety (Charney and Deutch, 1996; Gray and Bingaman, 1996). Thus, hypothalamic areas outside the PVN that contain CRH and/or CRH receptors may play a pivotal role in behavioral responses to stress and the manifestation of anxiety and depression.
Early in life, there is a delicate and critical balance of LHPA activity in the infant rat to maintain low stress hormone levels. From postnatal day (PND) 4 to 14, the adrenal response to mild stress is minimal; therefore, this period has been termed the stress-hyporesponsive period (SHRP) (for a review see Walker et al, 2001). Over the last two decades it has been evident that the neural substrate that orchestrates stress responses in the infant is not completely stress unresponsive. CRH (Dent et al, 2000a, 2000b), and ACTH and CORT responses (Walker et al, 2001; Walker and Dallman, 1993; Walker et al, 1991) are not absent in the infant rat, but rather the response is limited in magnitude, and the appearance of a response at the different levels of the axis is time and stressor specific. The animal model of prolonged maternal deprivation during the SHRP developed by Levine's group (for a review see de Kloet et al, 1988; Suchecki et al, 1995, 1993b) has been used by several laboratories to increase our understanding of the infant's stress physiology (Anisman et al, 1998; Avishai-Eliner et al, 1999; de Kloet et al, 1996; Eghbal-Ahmadi et al, 1999; Katz et al, 1996). When a mother is removed from the home cage, infant rats are exquisitely responsive to mild stressors and show persistent pituitary and adrenal hyper-responsiveness to stressors within the immediate postnatal period (Rosenfeld et al, 1992a; Stanton et al, 1988). CRH mRNA expression has been studied in the PVN in this paradigm, and has revealed that there is a rapid induction of heteronuclear and mature mRNA for CRH in both deprived and nondeprived animals within 15 min of an isotonic saline injection (Dent et al, 2000b). In both groups maximal levels are seen between 15 and 30 min, an increase that is followed by a decrease in the total CRH mRNA 60 min after the isotonic saline injection (Dent et al, 2000b). These findings indicate that during the SHRP, central brain elements are clearly responsive to environmental events, even though adrenal responses may be limited.
The distribution of brain CRH receptors changes as the animal develops (Avishai-Eliner et al, 1996; Eghbal-Ahmadi et al, 1998). Several brain regions have high expression of both CRHr1 and r2 receptors early in life, followed by a decline. In other regions, the expression of these genes is only evident during certain stages of development (Avishai-Eliner et al, 1996; Eghbal-Ahmadi et al, 1998). With the exception of the fact that maternal deprivation reduces CRHr2 gene expression in ventromedial hypothalamus (Eghbal-Ahmadi et al, 1997a, 1997b), little is known about the impact of early maternal separation on CRH receptor expression in the developing brain. Thus, one of the aims of the current study was to examine the maternal deprivation-induced changes on the 14-day-old developing rat CRH brain system.
Tricyclic antidepressants, whose modes of action include modulation of noradrenaline and serotonergic systems, have been shown to alter CRH in the PVN and hippocampal corticosteroid receptor expression in adult animal studies (Brady et al, 1991; López et al, 1998, 1997). Desipramine (DES), in particular, has selective noradrenaline reuptake inhibitor properties, and its administration to chronically stressed adult rats prevents the downregulation of hippocampal mineralocorticoid and serotonin 1a receptors, and the elevation of CORT levels that results from chronic stress (López et al, 1998). Although a direct effect of DES on these receptors is possible, reduction of circulating corticosteroids by DES is also important, since adrenalectomy also abolishes the stress-induced effects on these receptors (Chalmers et al, 1992, 1994; Kuroda et al, 1994; López et al, 1998; Mendelson and McEwen, 1992a, 1992b). We have shown that DES also limits the adrenocortical response in the maternally deprived infant pup (Vázquez et al, 2002). Since the adrenal response is reduced in the maternally deprived infant treated with DES, we hypothesized that alteration(s) in the CRH system that may result from prolonged maternal deprivation would also be prevented by this agent. Therefore, we performed in situ hybridization for CRH, CRHr1, and r2 mRNA using brain sections from a maternal deprivation study performed on 14-day-old animals treated with DES for 7 days prior to the deprivation event (Vázquez et al, 2002). We concentrated on specific brain areas that are rich in CRH neurons or that have reciprocal CRH connections, namely ventromedial hypothalamus (VMH), hippocampus, amygdala, and frontal cortex. We also quantified AVP and CRH mRNA in the hypothalamus (PVN). In addition, we performed a more detailed adrenocortical response time course in animals treated with DES during the second week of life and correlated the CRH molecule changes with the feedback profiles observed in the different treatment groups.
MATERIALS AND METHODS
Animals
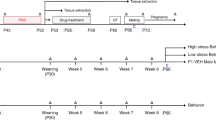
Animals were the product of Sprague–Dawley rats (Charles River, Chicago IL, USA). One male Sprague–Dawley rat was mated with two Sprague–Dawley females (trio-mating). On the calculated 18th day of gestation, the pregnant females were housed individually in polycarbonate cages (Nalgene, 24 × 45 × 20). On the 1st day of life, litters were culled to 12 healthy pups (six males, six females). The purpose of culling the litters to 12 pups was two-fold: (1) two male/female pairs could be assigned to the three treatment groups (see below) and (2) a total of 12 pups assured adequate nutrition since the number of pups matched the average number of nipples available for lactation. After this initial handling the cages were not disturbed in order to minimize disruption to the mother–pup interaction. The animals were kept under constant temperature (25±2°C) and lighting (12 h light, 12 h dark cycle) conditions. Rats were provided with rat chow and tap water ad libitum and maintained in accordance with the NIH Guidelines for the Care and Use of Laboratory Animals. The University of Michigan Animal Use Committee approved all animal protocols utilized. Figure 1 depicts the study design described below.
A time line of the treatment groups is presented. Outcome measures are performed at PND 14. Litters were culled to 12 pups at birth with equal male and female representation. On PND 7, two males and two females per litter were assigned to one of three groups: (1) Handled; (2) isotonic saline VEH; and (3) DES. On PND 13, these groups were further divided into NDEP or DEP condition, resulting in a total of six experimental groups. Study 1, Acute Challenge: 24 h after deprivation and at the equivalent time for the NDEP groups, one pair of animals per treatment group was killed (basal or 0 min). The remaining pairs were given an i.p. 3% saline injection and killed 60 min later. Study 2, Time Course of Acute Challenge: animals were treated in the same fashion. At 24 h after deprivation and at an equivalent time for the NDEP groups, one animal per treatment group was killed (0 min). The remaining animals were given an i.p. 3% saline injection and killed at 30, 60, and 120 min later. ISH—in situ hybridization.
Study 1: On the 7th day of life, the dams were removed from the cage and the litter was transported to a room adjacent to the colony. Two male and two female pups within each litter were weighed, measured, and assigned at random to one of the three treatment groups: (1) Handled; (2) vehicle (VEH); and (3) DES. In addition, the Handled, VEH, and DES pups were permanently marked with clipped ears (VEH and DES) or tail (Handled). VEH pups were administered a daily subcutaneous (s.c.) injection of 0.9% saline (0.01 ml/g body weight (BW)), while the DES pups received an s.c. injection of DES (10 g/kg/day, 0.01 ml/g BW). Pups in the Handled group were weighed daily, but did not receive injections. These treatments were administered at the same time each day from days 7 to 13 (4 h after lights ON). On the 13th day of life, after animals were injected with their respective treatments, the 12 litters originating from a trio-mating were randomly assigned to a maternally deprived (DEP, six litters) or nondeprived (NDEP, six litters) group.
Deprivation Procedure
The maternal deprivation started 4–6 h after lights ON, on PND 13. Deprived pups (DEP) are those whose dam was removed and the litter remained in the home cage. During the 24 h deprivation period the cages were placed on an electric heating pad, set at 30–33°C, in a room adjacent to and under the same temperature and lighting conditions as the main animal colony room. No food or water was available during this period of time. Nondeprived pups (NDEP) served as the comparison group. They were also moved to the room adjacent to the colony room, but were left undisturbed with their mothers until testing.
Testing Procedure
Testing was performed at the end of the deprivation period or at the equivalent time point for the NDEP animals. Within each litter one male and one female per treatment group were killed immediately (time 0). The remaining pups were weighed, given an intraperitoneal (i.p.) injection of 3% saline (0.01 ml/g BW), and returned to their cage until they were killed 1 h later.
Tissue Collection
Animals were killed by rapid decapitation. Trunk blood was collected in EDTA-treated precooled 1.5 ml vials at time 0 and time 60 min. The samples were kept cold until centrifugation. Plasma was separated and stored at −70°C until assayed for CORT by radioimmunoassay (RIA) (see below). Brains were rapidly removed, frozen in liquid isopentane, and stored at −80°C. Subsequently, they were sectioned in coronal plane at 12 μm on a cryostat and thaw mounted onto polylysine-coated microscope slides. Brain sections were stored at −80°C until processed for in situ hybridization (see below). The brain sections and plasma CORT levels collected constitute a common tissue and hormonal data core that has generated another report (Vázquez et al, 2002).
Study 2: Low CORT levels were observed at the 60 min time point in the DEP animals treated with DES (Vázquez et al, 2002). This suggested a blunting of the stress response. However, another possibility was that the LHPA axis in these DES-treated animals had matured to an adult quality where the 60 min time point was low because of an adequate termination of the stress response. To further explore the quality of the CORT response of the different treatment groups, we repeated the interlitter design described above. On the 14th day of life the 3% saline testing was performed at the end of the 24 h deprivation period or at an equivalent time point for the NDEP animals. A total of 18 litters were used (nine NDEP litters and nine DEP). Within each litter the animals were randomly assigned to four time points (0, 30, 60, and 120 min), irrespective of sex. The animals assigned to 0 min were weighed and killed immediately (0 min: NDEP, n=9; DEP, n=9 per treatment group). The remaining pups were given a 3% saline injection, returned to their cage that was divided into different areas, and killed at their assigned time point. The number of animals per time point, per treatment condition and maternal deprivation status, was nine. Trunk blood was collected in EDTA-treated precooled 1.5 ml vials. The samples were kept cold until centrifugation when plasma was separated and stored at −70°C until assayed by RIA.
Technical Methods
-
1
RIA: CORT was assayed with all groups represented using a RIA as previously described (Vázquez and Akil, 1992). The antibody crossreacts 2.2% with cortisol and less than 1% with other endogenous steroids. The detection limit is 1 pg/ml and the intra- and intercoefficient of variation is 2 and 3%, respectively. Results were analyzed by multifactor ANOVA considering age, sex, treatment, time, and maternal deprivation status. When differences were determined to be nonsignificant, the data were collapsed across the respective variable. The level of significance was set at p<0.05. Post hoc comparisons were made by using Fisher's protected least significant difference (PLSD).
-
2
Riboprobe design: CRH cRNA probe was produced from a 353 bp fragment derived from a rat cDNA clone (courtesy of R Thompson) that includes exon 2, the peptide region of the rat CRH gene. CRHr1 and r2 probe were derived from two pBluescript (SK+) plasmids containing a 415 and 461 bp fragment, respectively (kindly provided by TW Lovenberg). The CRHr1 fragment was the product of PCR amplification between transmembrane domains III and VII of the CRHr1 receptor. The CRHr2 probe is a unique product of the PCR reaction (alpha CRHr2 CRD23 clone) (Lovenberg et al, 1995a). Riboprobes were produced using either SP6 or T7 transcription systems in a standard labeling reaction mixture consisting of: 1 μg linearized plasmid, 5 × SP6 transcription buffer, 125 μCi 35S-UTP, 150 μM NTP's, 12.5 mM dithiothreitol, 20 U RNAse inhibitor, and 6 U of the appropriate polymerase. The reaction was incubated at 37°C for 90 min, and labeled probe was separated from free nucleotides over a Sephadex G50-50 column.
-
3
In situ hybridization: Sections were removed from storage at −80°C and placed directly into 4% buffered paraformaldehyde at room temperature. After 60 min, slides were rinsed in isotonic phosphate-buffered saline and treated with proteinase K (1 μg/ml in 100 mM Tris/HCl, pH 8.0) for 10 min at 37°C. Subsequently, sections underwent successive washes in water (1 min), 0.1 M triethanolamine (pH 8.0, plus 0.25% acetic anhydride) for 10 min and 2 × SSC (0.3 mM NaCl, 0.03 mM sodium citrate, pH 7.2) for 5 min. Sections were then dehydrated through graded alcohols and air-dried. Postfixed sections were hybridized with 1.0 × 106 dpm [35S]UTP-labeled riboprobes in a hybridization buffer containing 50% formamide, 10% dextran sulfate, 3 × SSC, 50 mM sodium phosphate buffer (pH 7.4), 1 × Denhardt's solution, 0.1 mg/ml yeast tRNA, and 10 mM dithiothreitol in a total volume of 25 μl. The probe was applied to sections on a glass coverslip and hybridized overnight at 55°C. The following day, sections were washed in 2 × SSC for 5 min and then treated with RNAse A (200 μg/ml in 10 mM Tris/HCl, pH 8.0, containing 0.5 M NaCl) for 60 min at 37°C. Subsequently, sections were washed in 2 × SSC for 5 min, 1 × SSC for 5 min, 0.5 × SSC for 60 min at hybridization temperature, 0.5 × SSC at room temperature for 5 min, and then dehydrated in graded alcohols and air-dried. For signal detection, sections were placed on Kodak XAR-5 X-ray film and exposed for several days (depending on the probe) at room temperature.
Analyses
-
1
Microdensitometric analysis: The autoradiograms generated from the in situ hybridization were analyzed using an automated image analysis system (Dage camera, NIH IMAGE). To correct for film nonlinearity, 14C-methylmethacrylate standards were used and the in situ hybridization assays for each probe were carried out at the same time for all molecules analyzed. Brain regions for the quantification of the different molecules were identified using the Paxinos and Watson neuroanatomical rat atlas. For the hippocampus quantification, subfields corresponding to CA1, CA2, CA3/4, and dentate gyrus were digitized from a given section. For all quantification, the background labeling was measured from a corresponding internal area of each section. The person analyzing the images was not aware of the treatment conditions under analysis. Four sections per animal were analyzed. The mean of six to eight measurements was used as the individual value for a particular area in an animal.
-
2
Statistical analyses: A multivariate ANOVA was used for the simultaneous analysis of sex, anatomical area, treatments during the second week of life, and maternal deprivation. All data are expressed as means±SEM, and significance was accepted at p<0.05. No gender differences were found for plasma hormones, AVP, CRH, CRHr1, and r2; therefore, the data were collapsed across these variables. No acute stress effect was found for AVP, CRH, CRHr1, and r2. Therefore, the 0 and 60 min data were collapsed for the in situ hybridization analyses of these molecules. When the multifactorial ANOVA revealed an overall effect for anatomical region as well as for treatment and/or deprivation, the statistical significance between groups was determined by ANOVA for each anatomical region, followed by Fisher's PLSD test comparisons.
Plasma CORT for the time course study was analyzed using a multivariate ANOVA analysis that considered sex, time after the 3% saline challenge, treatment groups, and maternal deprivation. In addition, the overall CORT release was also assessed for each of the CORT profiles calculating area under each curve (AUC) using the least-squares method. Once this calculation was obtained, the statistical significance between groups was determined by ANOVA, followed by Student–Newman–Keuls test comparisons.
RESULTS
Adrenocortical Response
Table 1 depicts the acute CORT response 60 min after the 3% saline challenge. These data have been previously reported (Vázquez et al, 2002), and are presented here to facilitate the interpretation of all the CRH-related data reported herein. A multifactorial ANOVA did not reveal a sex effect (F=1.58, p=0.21). Significant differences were detected for maternal deprivation (F=64.2, p<0.0001), time (F=176.5, p<0.0001), with a maternal deprivation by injection treatment interaction (F=5.3, p<0.001). As previously reported (Rosenfeld et al, 1992a; Stanton et al, 1988), prolonged maternal deprivation resulted in a significant increase of basal CORT levels (time 0, NDEP vs DEP, F=74.3, p<0.001). Plasma CORT levels were also significantly elevated 1 h after the 3% saline injection (F=66.5, p<0.0001) and the DEP VEH-treated animals had a trend to higher CORT levels compared to DEP Handled animals. In contrast, 60 min after the 3% saline injection, DEP animals treated with DES had decreased CORT levels compared to the other maternally deprived groups (group by treatment interaction, F=5.5, p<0.001). In addition, the NDEP DES-treated animals showed a modest but significant CORT increase 60 min after the 3% saline challenge (60 min, F=5.5, p<0.01). This was significantly different from the NDEP Handled and VEH-treated animals that did not respond to the 3% saline challenge. Thus, DES appears to have a differential effect, depending on the deprivation status of the animal. While DES decreased the stress-induced response in DEP, this agent appears to have facilitated the activation of the adrenocortical response to 3% saline in nondeprived animals.
To further examine the quality of the adrenocortical response in these various treatments, we sampled animals at different time points after the administration of the 3% saline challenge. This is shown in Figure 2a and b. A three-way ANOVA revealed no sex effect (F=0.89, p=0.35). Subsequent analysis showed significant differences for deprivation (F=77.8, p<0.0001) and time (F=45.3, p<0.0001), with a deprivation by treatment interaction (F=73.2, p<0.01). NDEP animals from the various treatment groups had different patterns of adrenocortical response (see Figure 2a). Significant differences were observed at 60 min. Specifically, at 60 min after the 3% saline injection, the NDEP DES-treated animals showed increased CORT levels compared to NDEP VEH (p<0.0001) and NDEP Handled animals (p<0.05). By 120 min, the NDEP DES-treated animals had low basal CORT levels indicating inhibition of the stress response. In contrast, the NDEP Handled animals exhibited a low, but sustained CORT secretion, compared to the other groups (120 min: Handled vs VEH, Handled vs DES, p<0.0001).
Plasma CORT levels at 30, 60, and 120 min after a 3% saline challenge in NDEP (a) and DEP (b) 14-day-old animals subjected to 7 days of handling (Handled), isotonic saline vehicle injections (VEH), or DES (10 mg/kg/day). Data are presented as mean±SE, *significantly different from Handled (p<0.05). Number of animals: both, DEP, and NDEP groups: Handled, VEH, and DES, each have nine animals per time point.
The DEP animals also displayed different patterns of CORT secretion following the 3% saline challenge (Figure 2b). Significant differences were observed at 30 min, at which time DES- and VEH-treated animals had significantly higher CORT levels compared to the DEP Handled group (p<0.0001). By 60 min, the DEP animals treated with DES had returned to baseline (DES vs VEH, p<0.001), whereas DEP animals treated with VEH and those that received handling remained elevated. At 120 min, the DEP Handled animals continued to have sustained CORT release, while the VEH-treated animals had returned to baseline (Handled vs VEH, p<0.001; Handled vs DES, p<0.001).
We also analyzed total adrenal CORT release using the data presented in Figure 2. A multifactorial ANOVA of the AUC revealed no sex effect (F=0.088, p=0.77). Significant differences were detected for deprivation (F=86.72, p<0.0001) and treatments (F=20.42, p<0.0001). Interactions between the variables were not detected. Handled animals consistently showed high total CORT release in both NDEP and DEP animals (AUC: NDEP Handled=225.45±66.9 μl; VEH=59.55±16.7 μl; DES=73.15±26.6 μl; DEP Handled=753.7±34.6 μl; VEH=294.7±73.6 μl; DES=448.1±47.6 μl; Handled vs VEH and Handled vs DES, p<0.05). Therefore, while it is clear that deprivation causes an enhanced and prolonged adrenocortical response to stress, both chronic DES and VEH treatments promote adrenocortical feedback in the developing animal, although at different time frames. DES treatment leads to quick ‘shutdown’ of the CORT response (60 min), whereas VEH treatment inhibits the adrenocortical response 1 h later (120 min after challenge).
Brain Elements
We quantified brain areas that are part of the LHPA axis, in addition to areas that are rich in CRH neurons or that have reciprocal CRH connections. The multivariate ANOVA analyses that considered the brain region, maternal deprivation condition, treatment (Handled, VEH, or DES), time before (0 min) and after (60 min) 3% saline challenge, and sex did not reveal a sex effect (AVP: F=2.2, p=0.09; CRH: F=1.0, p=0.31; CRHr1: F=1.6, p=0.22; CRHr2: F=2.17, p=0.14). This is consistent with other studies using a similar paradigm early in life (Dent et al, 2000a, 2000b, 2001; Eghbal-Ahmadi et al, 1999; Vázquez et al, 2000; Vázquez et al, 1996). An acute 3% saline stress effect was found for CRHr1 (CRHr1: F=11.8, p=0.0007). All other molecules were not regulated at 60 min by this acute stress challenge (AVP: F=2.35, p=0.09; CRH: F=0.07, p=0.92; CRHr2: F=0.02, p=0.88). Dictated by these analyses, the data were collapsed across these variables for the appropriate molecules. An illustration of the in situ hybridization signal for the molecules that were investigated in this study is presented in Figure 3.
A representative photomicrograph depicting the messenger RNA of the different molecules analyzed in the study (irrespective of treatment group). Panels are as follows: (a) CRH mRNA; (b) AVP mRNA; (c) CRHr1 mRNA; (d) CRHr2 mRNA. Abbreviations: paraventricular nucleus of the hypothalamus (PVN), Amygdala (AM).
CRH and AVP mRNA Levels
Figure 4 depicts CRH mRNA (panel a) and AVP mRNA (panel b) in the PVN of all groups studied. A three-way ANOVA revealed that the CRH mRNA expression in the PVN was significantly decreased in the deprived animals irrespective of the treatment group (deprivation, F=11.4, p<0.001). The CRH mRNA downregulation observed in the DEP animals was not prevented by DES treatment (treatment, F=0.20, p=0.81). In contrast, AVP mRNA expression was increased in the DEP animals (three-way ANOVA, deprivation, F=8.7, p<0.001). The upregulation observed in the DEP animals was not prevented by DES (treatment, F=0.62, p=0.53). Interactions between the variables were not detected for either molecule in this region.
CRH mRNA levels were also analyzed in the three isodense bands visualized across the prefrontal cortical layers (Figure 5a). Significant upregulation was evident with deprivation in those animals that received chronic injections (deprivation, F=13.1, p<0.0001; see Figure 5a which depicts all cortical layers). There were no treatment (F=2.2, p=0.11), or treatment by deprivation effects (F=3.02, p=0.06). DES treatment did not alter these patterns. CRH mRNA levels were not affected by deprivation (F=0.001, p=0.98) or treatments (F=0.9, p=0.92) in the central nucleus of the amygdala (Ce). This is shown in Figure 5b.
CRHr1 mRNA Levels
CRHr1 mRNA expression quantification is depicted in Figure 6. In the prefrontal cortex CRHr1 mRNA was upregulated with deprivation (F=7.9, p<0.001, see Figure 6a). However, we did not detect treatment (F=0.33, p=0.71) or treatment : deprivation interactions (F=0.09, p=0.9). Similarly, in the hypothalamic region there was no deprivation (F=0.03, p=0.86) or treatment effect (F=0.7, p=0.5; see Figure 6b). The hippocampal CRHr1 mRNA expression analysis by three-way ANOVA revealed a subfield effect (F=176.2, p<0.0001). Therefore, we analyzed each of these independently. Although CRHr1 expression was different from region to region, no deprivation (F=2.0, p=0.12) or DES treatment effects (F=2.3, p=0.1) were found (see eg CA1 in Figure 6c).
Densitometric analysis of CRHr1 mRNA in regions that are part of the LHPA axis and stress CRH brain circuits. (a) Prefrontal cortex, (b) hypothalamic PVN, (c) hippocampus CA1 pyramidal cell region, (d) medial amygdala, (e) basolateral amygdala, (f) central amygdala. *NDEP vs DEP, p<0.001, n=12 for each group. Error bars not shown are too small to be graphed.
The basolateral (BL), medial (Me) and central (Ce) nuclei of the amygdala were evaluated. As can be seen from Figure 6d–f deprivation caused significant regulation of the CRHr1 mRNA in two of the three amygdala nuclei examined (Me (F=5.3, p<0.05) and Ce (F=7.9, p<0.001). No treatment or treatment : deprivation interactions were detected (treatment: Me—F=2.7, p=0.07; Ce—F=0.5, p=0.62; treatment : deprivation: Me—F=0.6, p=0.9; Ce—F=0.16, p=0.8). No significant effects were detected in the BL nucleus (Figure 6d).
CRHr2 mRNA Levels
CRHr2 mRNA levels are presented in Figure 7. In the prefrontal cortex, CRHr2 mRNA levels followed a profile similar to CRHr1 mRNA levels. Deprivation caused upregulation of CRHr2 mRNA levels in this region (F=20.2, p<0.0001, see Figure 7a). No treatment effects were detected (F=1.0, p=0.37). However, DES prevented the upregulation of CRHr2 in this structure (treatment : deprivation, F=7.9, p<0.05). In contrast, the ventromedial hypothalamic nucleus (VMH) CRHr2 mRNA expression was significantly decreased by maternal deprivation (deprivation, F=11.8, p<0.0001; see Figure 7b). Yet DES treatment again prevented the maternal deprivation effect observed in this region (treatment by condition, F=3.6, p<0.01).
Densitometric analysis of CRHr2 mRNA in structures that are part of the LHPA axis and stress CRH brain circuits. (a) Prefrontal cortex, (b) hypothalamic VMH, (c) central amygdala, (d) hippocampus CA1 pyramidal cell region, (e) hippocampus CA2 pyramidal cell region, (f) hippocampus CA3-4 pyramidal cell region. DES prevented the changes induced by maternal deprivation. *NDEP vs DEP, p<0.001, n=12 for each group. Error bars not shown are too small to be graphed.
The analyses of the CRHr2 mRNA expression in amygdala revealed a deprivation effect in the Ce only (see Figure 7c, F=8.6, p<0.001). No treatment effects were detected (F=1.7, p=0.18), but again, DES prevented the downregulation caused by deprivation (treatment : deprivation, F=3.8, p<0.05). No significant changes in CRHr2 mRNA were detected in the ME and BL nuclei (data not shown).
In the hippocampus, the analyses revealed a significant effect of subfield (F=165.8, p<0.0001). The individual analysis of the regions showed significant upregulation of CRHr2 mRNA by deprivation in all pyramidal cell regions (see Figure 7d–f; CA1, F=10.2, p<0.001; CA2, F=8.9, p=0.001; CA3–4, F=5.7, p=0.01; DG, F=0.33, p=0.56). DES prevented the upregulation observed in CA1 and CA2 (treatment by deprivation, CA1, F=4.7, p<0.01; CA2, F=5.6, p=0.001). In the CA3–4 subfield, chronic isotonic saline injection treatment interacted with maternal deprivation (CA3–4, F=3.7, p<0.01; see Figure 7f). In this region only the VEH-injected group showed an upregulation of CRHr2 after deprivation. Again, DES prevented this effect (CA3–4, F=4.4, p<0.01).
DISCUSSION
For many years it has been known that 24 h of maternal deprivation causes increased basal and stress-induced CORT levels in the infant rat (Stanton et al, 1988). The present series of studies focused on the 14-day-old animal, utilizing three different treatment modalities in an interlitter design, namely handling, isotonic saline VEH injection, and DES administration during the second week of life. We specifically evaluated the immediate effects of maternal deprivation on critical molecules linked to the modulation of the extrahypothalamic CRH system and the effect of DES treatment on the maternal deprivation response. Our results complement previous studies that indicate that maternal deprivation activates brain structures in a unique manner (Smith et al, 1997; van Oers et al, 1998b). We found that the alterations mediated by maternal deprivation are molecule specific, specific for particular brain structures, and with patterns that suggest integration by circuits while they are undergoing development. In addition, the prior experience of a chronic isotonic saline injection alters some aspects of the CRH molecule response, which is reflected mainly in DEP. Our results also indicate that DES selectively modulates the CRHr2 receptor, a finding that suggests a unique mechanism of action by tricyclic antidepressants on the urocortin-related CRH brain system. Finally, it is also evident from our study that the adrenal response of the nondeprived and deprived 14-day-old rat is differentially altered by these treatments. Specifically, we found both a stress response enhancement and a more efficient negative feedback with chronic isotonic saline injection and DES treatments.
The time course of the adrenal response revealed that the chronic intermittent stress of the isotonic injection caused an enhancement of the adrenocortical stress response to the 3% saline challenge at 30 min that was specific to the deprived animals in the VEH- and DES-treated groups. It is possible that the specific handling experience received by our comparison group may have accentuated these differences, since brief daily handling also influences the development of the LHPA, causing blunting of the LHPA in adult animals (Levine, 1957). In addition, two other treatments may have facilitated the adrenocortical response and CRH mRNA changes in the prefrontal cortex (see below) observed in the VEH- and DES-treated animals. The animals were subjected to the stress of ear/tail clipping on PND 7, and on PND 14 we used as a challenge a concentration of saline that may be considered a noxious stimuli (3% saline). Even with these caveats, the enhanced adrenocortical response to the 3% saline challenge observed in the deprived Handled-, VEH-, and DES-treated groups is consistent with previous studies that have shown that maternal deprivation enhances adrenal sensitivity to ACTH treatment in deprived animals compared to unhandled pups (Levine et al, 1991; Rosenfeld et al, 1992b; Walker, 1995). Similarly, maternal deprivation enhances adrenocortical responses to novel environments (Suchecki et al, 1993a). In spite of the enhanced CORT activation observed, we did not find upregulation of CRH mRNA in the hypothalamus 60 min after the test challenge. Instead, we found a downregulation. This is not an unexpected finding since it is known that the induction of CRH transcription in the PVN is rapid, within 15 min in both deprived and nondeprived animals in response to the mild stimulus of isotonic saline injection (Dent et al, 2000b), and that after 60 min, CRH mRNA downregulation is observed (Dent et al, 2000a). Our studies evaluated the CRH gene expression at 60 min after a hypertonic saline challenge. Therefore, earlier time points may be necessary to uncover CRH upregulation. However, we observed an enhanced AVP gene expression at 60 min after the acute challenge, an effect that probably reflects both the magnitude of the stress and osmolarity changes caused by the hypertonic saline injection (Harbuz et al, 1994). Ultimately, as shown in other studies (Dent et al, 2000a), the AVP's upregulation, shown here, likely contributed to the increased biosynthesis and synergy with CRH that elicited an enhanced stress response in DEP in our studies.
CRH-related gene expression changes in the amygdala and prefrontal cortical areas have been associated with mechanisms that may underlie enhancement or facilitation of the LHPA response (Bhatnagar and Dallman, 1998; Herman and Cullinan, 1997; Herman et al, 1996). We found that the 14-day-old maternal-deprived animals subjected to daily isotonic saline injections for 7 days showed changes in these areas. The effects were regionally specific and not unidirectional. Maternal deprivation resulted in a downregulation of CRHr1 mRNA levels in the central nucleus of the amygdala, suggesting an increased occupation of this receptor. In contrast, daily isotonic saline injections in the VEH-treated deprived animals potentiated CRH mRNA synthesis in the prefrontal cortex without affecting CRH receptor mRNA expression in this area. Thus, it is possible that these site-specific CRH and CRHr1 gene expression changes may be linked to the enhancement of the CORT response observed in deprived Handled animals and in the deprived animals that received daily isotonic saline injections.
We also found that adrenocortical feedback inhibition was enhanced by our treatments. Others have reported that although circulating levels of CORT are low during the first two weeks of life, modest but significant inhibition of ACTH secretion by CORT is observed in neonatal rats (van Oers et al, 1998a; Walker and Dallman, 1993). In spite of this capacity to inhibit ACTH secretion, the adrenocortical response remains elevated for a prolonged period, as shown in an elegant work by Suchecki et al (1995). These investigators found that the time course of the stress response in the deprived animal is characterized by CORT and ACTH that remain persistently elevated through a 120 min period following an isotonic saline injection (Suchecki et al, 1995). This persistent elevation of CORT and ACTH is also seen in the NDEP animal once it exhibits an adrenocortical response to stress at 15 days of age (Suchecki et al, 1995). The inhibition of the adrenocortical stress response is achieved by 25 days of age when a more efficient ACTH inhibition and metabolic clearance is possible (Goldman et al, 1973; Vázquez, 1998; Vázquez and Akil, 1993; Vázquez et al, 1997). We found that NDEP and DEP animals treated with chronic isotonic saline injections had low CORT levels at 120 min, and that DES treatment resulted in a more efficient inhibition of the adrenocortical response by 60 min after the 3% saline challenge. Total CORT release, evaluated by calculating the area under the curve, was comparable between deprived animals subjected to chronic isotonic saline injections and DES treatment, but significantly different from deprived, Handled animals in each respective group. DES, however, does not alter the elevated basal CORT levels seen with 24 h deprivation, suggesting that these chronic treatments have an effect on stimulated CORT release.
It is possible that CRH receptors are involved in the development of an efficient inhibition of the stress activation. In particular, CRHr2 may be a key regulator of the termination phase of the stress response. This is supported by the fact that CRHr2-deficient mice exhibit abnormal recovery from activation of the LHPA axis. CRHr2-deficient mice have CORT levels that are significantly elevated 90 min poststress compared to wild-type mice (Bale et al, 2000; Coste et al, 2000). Therefore, the selective prevention of the CRHr2 changes by DES in our study may be of particular importance in modifying the recovery phase of the LHPA response in the deprived infant pup. This may be a direct effect on the CRHr2 receptors or an effect mediated through changes in urocortin peptides (urocortin 1, 2, and 3) that preferentially bind to CRHr2 receptors (for a review see Chalmers et al, 1996; Lewis et al, 2001). Although the developmental profiles for the urocortins are yet to be described, based on adult animal anatomical distribution profiles and suggested modulation of neuroendocrine function related to stress (Lewis et al, 2001), urocortin 3 is an attractive candidate to explain our findings. Urocortin 3 terminals reach into the amygdala, and project from there to the hypothalamus and other limbic forebrain structures, a distribution pattern that coincides with the CRHr2 effects described here (Lewis et al, 2001).
It is of particular interest that the tricyclic antidepressant, DES, a potent noradrenaline reuptake inhibitor, altered the stress response and also prevented the maternal deprivation-induced changes of CRHr2 mRNA in all brain regions. A number of terminal projections of the forebrain noradrenaline systems, namely PVN, the bed nucleus of the stria terminalis, and the central nucleus of the amygdala, are areas where noradrenaline stimulates CRH release (Koob, 1999). Perhaps DES may be directly or indirectly modulating CRH expression in these brain sites, which in turn may cause the site-specific CRHr2 effect seen in the deprived animals. It is not known if increases in noradrenaline at specific synaptic sites where urocortins are expressed may also stimulate the synthesis and release of these peptides at their projection sites. Although the specific mechanism of action remains to be explored, the selective modulation of the CRHr2 receptor by DES suggests a unique mechanism of action by tricyclic antidepressants on the CRH brain system during development. Such a feed-forward system may be particularly important in infant response to environmental challenge.
In conclusion, maternal deprivation has an impact on CRH-related molecules in limbic structures and cortical areas. The effects are specific within the structures and are also specific for the molecules involved, suggesting neuronal interactions between limbic efferent pathways. We have also shown that another stressor, chronic isotonic saline injection, is capable of modifying the stress response, and altering the expression of some of these same molecules. In some instances, the chronic isotonic saline injection potentiated the effects of maternal deprivation. These results indicate that, in addition to maternal influences, other aversive experiences are capable of affecting the developing brain. Furthermore, the interactions of these events add complexities to the overall consequences of stress in brain neurobiology and behavior. The fact that DES administration was able to prevent some of the changes, but not others, suggests that several neurotransmitter systems are involved in this response. It also suggests that we should be able to design pharmacological strategies to prevent, at least partially, the neurobiological changes that may follow adverse early experience.
References
Anisman H, Zaharia MD, Meaney MJ, Merali Z (1998). Do early-life events permanently alter behavioral and hormonal responses to stressors? Int J Dev Neurosci 16: 149–164.
Avishai-Eliner S, Hatalski CG, Tabachnik E, Eghbal-Ahmadi M, Baram TZ (1999). Differential regulation of glucocorticoid receptor messenger RNA (GR-mRNA) by maternal deprivation in immature rat hypothalamus and limbic regions. Brain Res Dev Brain Res 114: 265–268.
Avishai-Eliner S, Yi SJ, Baram TZ (1996). Developmental profile of messenger RNA for the corticotropin-releasing hormone receptor in the rat limbic system. Brain Res Dev Brain Res 91: 159–163.
Bale TL, Contarino A, Smith GW, Chan R, Gold LH, Sawchenko PE et al (2000). Mice deficient for corticotropin-releasing hormone receptor-2 display anxiety-like behaviour and are hypersensitive to stress. Nat Genet 24: 410–414.
Bhatnagar S, Dallman M (1998). Neuroanatomical basis for facilitation of hypothalamic–pituitary–adrenal responses to a novel stressor after chronic stress. Neuroscience 84: 1025–1039.
Brady LS, Whitfield HJ, Fox RJ, Gold PW, Herkenham M (1991). Long-term antidepressant administration alters corticotropin-releasing hormone, tyrosine hydroxylase, and mineralocorticoid receptor gene expression in rat brain. J Clin Invest 87: 831–837.
Chalmers DT, Kwak SP, Mansour A, Akil H, Watson SJ (1992). Corticosteroids regulate brain hippocampal 5-HT1A receptor mRNA expression. J Neurosci 13: 914–923.
Chalmers DT, López JF, Vazquez DM, Akil H, Watson SJ (1994). Regulation of hippocampal 5-HT1A receptor gene expression by dexamethasone. Neuropsychopharmacology 10: 215–222.
Chalmers DT, Lovenberg TW, Grigoriadis DE, Behan DP, De Souza EB (1996). Corticotropin-releasing factor receptors: from molecular biology to drug design. Trends Pharmacol Sci 17: 166–172.
Charney DS, Deutch A (1996). A functional neuroanatomy of anxiety and fear: implications for the pathophysiology and treatment of anxiety disorders. Crit Rev Neurobiol 10: 419–446.
Coste SC, Kesterson RA, Heldwein KA, Stevens SL, Heard AD, Hollis JH et al (2000). Abnormal adaptations to stress and impaired cardiovascular function in mice lacking corticotropin-releasing hormone receptor-2. Nat Genet 24: 403–409.
Dallman MF (2000). Moments in time—the neonatal rat hypothalamo-pituitary–adrenal axis [editorial]. Endocrinology 141: 1590–1592.
de Kloet ER, Rosenfel P, Van Eekelen JAM, Sutanto W, Levine S (eds) (1988). Stress, glucocorticoids and development. Progress in brain research. In: Boer GJ, Feenstra MGP, Swaab DF, Van Haaren F (eds), Vol. 73. Elsevier: Amsterdam, 101–120.
de Kloet ER, Rots NY, Cools AR (1996). Brain-corticosteroid hormone dialogue: slow and persistent. Cell Mol Neurobiol 16: 345–356.
Dent GW, Okimoto DK, Smith MA, Levine S (2000a). Stress-induced alterations in corticotropin-releasing hormone and vasopressin gene expression in the paraventricular nucleus during ontogeny. Neuroendocrinology 71: 333–342.
Dent GW, Smith MA, Levine S (2000b). Rapid induction of corticotropin-releasing hormone gene transcription in the paraventricular nucleus of the developing rat. Endocrinology 141: 1593–1598.
Dent GW, Smith MA, Levine S (2001). Stress-induced alterations in locus coeruleus gene expression during ontogeny. Brain Res Dev Brain Res 127: 23–30.
Eghbal-Ahmadi M, Avishai-Eliner S, Hatalski CG, Baram TZ (1999). Differential regulation of the expression of corticotropin-releasing factor receptor type 2 (CRF2) in hypothalamus and amygdala of the immature rat by sensory input and food intake. J Neurosci 19: 3982–3991.
Eghbal-Ahmadi M, Hatalski CG, Avishai-Eliner S, Baram TZ (1997a). Corticotropin releasing factor receptor type II (CRF2) messenger ribonucleic acid levels in the hypothalamic ventromedial nucleus of the infant rat are reduced by maternal deprivation [published erratum appears in Endocrinology 1998 Jan; 139(1):136]. Endocrinology 138: 5048–5051.
Eghbal-Ahmadi M, Hatalski CG, Avishai-Eliner S, Baram TZ (1997b). Corticotropin releasing factor receptor type II (CRF2) messenger ribonucleic acid levels in the hypothalamic ventromedial nucleus of the infant rat are reduced by maternal deprivation. Endocrinology 138: 5048–5051.
Eghbal-Ahmadi M, Hatalski CG, Lovenberg TW, Avishai-Eliner S, Chalmers DT, Baram TZ (1998). The developmental profile of the corticotropin releasing factor receptor (CRF2) in rat brain predicts distinct age-specific functions. Brain Res Dev Brain Res 107: 81–90.
Goldman L, Winget C, Holinshead G, Levine S (1973). Postweaning development of negative feedback in the pituitary–adrenal system of the rat. Neuroendocrinology 12: 188–211.
Gray TS, Bingaman EW (1996). The amygdala: corticotropin-releasing factor, steroids and stress. Crit Rev Neurobiol 10: 155–168.
Harbuz MS, Jessop DS, Lightman SL, Chowdrey HS (1994). The effects of restraint or hypertonic saline stress on corticotrophin-releasing factor, arginine vasopressin, and proenkephalin A mRNAs in the CFY, Sprague–Dawley and Wistar strains of rat. Brain Res 667: 6–12.
Herman JP, Cullinan WE (1997). Neurocircuitry of stress: central control of the hypothalamo-pituitary–adrenocortical axis. Trends Neurosci 20: 78–84.
Herman JP, Prewitt CM, Cullinan WE (1996). Neuronal circuit regulation of the hypothalamo-pituitary–adrenocortical stress axis. Crit Rev Neurobiol 10: 371–394.
Katz LM, Nathan L, Kuhn CM, Schanberg SM (1996). Inhibition of GH in maternal separation may be mediated through altered serotonergic activity at 5-HT2A and 5-HT2C receptors. Psychoneuroendocrinology 21: 219–235.
Koob GF (1999). Corticotropin-releasing factor, norepinephrine, and stress. Biol Psychiatry 46: 1167–1180.
Kuroda Y, Watanabe Y, Albeck DS, Hastings NB, McEwen BS (1994). Effects of adrenalectomy and type I or type II glucocorticoid receptor activation on 5-HT1A and 5-HT2 receptor binding and 5-HT transporter mRNA expression in rat brain. Brain Res 648: 157–161.
Ladd CO, Owens MJ, Nemeroff CB (1996). Persistent changes in corticotropin-releasing factor neuronal systems induced by maternal deprivation. Endocrinology 137: 1212–1218.
Levine S (1957). Infantile experience and resistance to physiological stress. Science 126: 405.
Levine S, Huchton DM, Wiener SG, Rosenfeld P (1991). Time course of the effect of maternal deprivation on the hypothalamic–pituitary–adrenal axis in the infant rat. Dev Psychobiol 24: 547–558.
Lewis K, Li C, Perrin MH, Blount A, Kunitake K, Donaldson C et al (2001). Identification of urocortin III, an additional member of the corticotropin-releasing factor (CRF) family with high affinity for the CRF2 receptor. Proc Natl Acad Sci USA 98: 7570–7575.
López JF, Chalmers D, Little KY, Watson SJ (1998). Regulation of 5HT1a receptor, glucocorticoid and mineralocorticoid receptor in rat and human hippocampus: implications for the neurobiology of depression. Biol Psychiatry 43: 547–573.
López JF, Vazquez DM, Chalmers DT, Watson SJ (1997). Regulation of 5-HT receptors and the hypothalamic–pituitary–adrenal axis: implications for the neurobiology of suicide. Ann NY Acad Sci 386: 106–134.
Lovenberg TW, Liaw CW, Grigoriadis DE, Clevenger W, Chalmers DT, De Souza EB (1995a). Cloning and characterization of a functionally distinct corticotropin-releasing factor receptor subtype from rat brain. Proc Natl Acad Sci USA 92: 836–840.
Mendelson SD, McEwen BS (1992a). Autoradiographic analyses of the effects of adrenalectomy and corticosterone on 5-HT1A and 5-HT1B receptors in the dorsal hippocampus and cortex of the rat. Neuroendocrinology 55: 444–450.
Mendelson SD, McEwen BS (1992b). Quantitative autoradiographic analyses of the time course and reversibility of corticosterone-induced decreases in binding at 5-HT1A receptors in rat forebrain. Neuroendocrinology 56: 881–888.
Perrin MH, Vale WW (1999). Corticotropin releasing factor receptors and their ligand family. Ann NY Acad Sci 885: 312–328.
Rosenfeld P, Suchecki D, Levine S (1992a). Multifactorial regulation of the hypothalamic–pituitary–adrenal axis during development. Neurosci Biobehav Rev 16: 553–568.
Rosenfeld P, Wetmore JB, Levine S (1992b). Effects of repeated maternal separations on the adrenocortical response to stress of preweanling rats. Physiol Behav 52: 787–791.
Smith MA, Kim SY, van Oers HJ, Levine S (1997). Maternal deprivation and stress induce immediate early genes in the infant rat brain. Endocrinology 138: 4622–4628.
Stanton ME, Gutierrez YR, Levine S (1988). Maternal deprivation potentiates pituitary–adrenal stress responses in infant rats. Behav Neurosci 102: 692–700.
Suchecki D, Mozaffarian D, Gross G, Rosenfeld P, Levine S (1993a). Effects of maternal deprivation on the ACTH stress response in the infant rat. Neuroendocrinology 57: 204–212.
Suchecki D, Nelson DY, Van Oers H, Levine S (1995). Activation and inhibition of the hypothalamic–pituitary–adrenal axis of the neonatal rat: effects of maternal deprivation [published erratum appears in Psychoneuroendocrinology 1995; 20: 677]. Psychoneuroendocrinology 20: 169–182.
Suchecki D, Rosenfeld P, Levine S (1993b). Maternal regulation of the hypothalamic–pituitary–adrenal axis in the infant rat: the roles of feeding and stroking. Dev Brain Res 75: 185–192.
van Oers HJ, de Kloet ER, Li C, Levine S (1998a). The ontogeny of glucocorticoid negative feedback: influence of maternal deprivation. Endocrinology 139: 2838–2846.
van Oers HJ, de Kloet ER, Whelan T, Levine S (1998b). Maternal deprivation effect on the infant's neural stress markers is reversed by tactile stimulation and feeding but not by suppressing corticosterone. J Neurosci 18: 10171–10179.
Vaughan J, Donaldson C, Bittencourt J, Perrin MH, Lewis K, Sutton S et al (1995). Urocortin, a mammalian neuropeptide related to fish urotensin I and to corticotropin-releasing factor. Nature 378: 287–292.
Vázquez DM (1998). The Curt Richter 1997 Award: stress and the developing limbic-hypothalamic–pituitary–adrenal axis. Psychoneuroendocrinology 23: 663–700.
Vázquez DM, Akil H (1992). Development of pituitary pro-opiomelanocortin gene and peptide expression: characterization and effect of repeated intermittent maternal isolation. Neuroendocrinology 56: 320–330.
Vázquez DM, Akil H (1993). Pituitary–adrenal response to ether vapor in the weanling animal: characterization of the inhibitory effect of glucocorticoids on adrenocorticotropin secretion. Pediatr Res 34: 646–653.
Vázquez DM, Eskandari R, Levine S, López JF (2002). Brain serotonin receptor system in the stressed infant rat: implications for vulnerability to substance abuse. Psychoneuroendocrinology 27: 245–272.
Vázquez DM, López JF, Van Hoers H, Watson SJ, Levine S (2000). Maternal deprivation regulates serotonin 1A and 2A receptors in the infant rat. Brain Res 855: 76–82.
Vázquez DM, Morano MI, Taylor L, Akil H (1997). Kinetics of radiolabeled adrenocorticotropin hormone in infant and weanling rats. J Neuroendocrinol 9: 529–536.
Vázquez DM, Van Dours H, Levine S, Akil H (1996). Regulation of the glucocorticoid and mineralocorticoid receptor mRNA in the hippocampus of the maternally deprived infant rat. Brain Res 731: 79–90.
Walker CD (1995). Chemical sympathectomy and maternal separation affect neonatal stress responses and adrenal sensitivity to ACTH. Am J Physiol 268: R1281–R1288.
Walker CD, Anand KJS, Plotsky PM (2001). Development of the hypothalamic–pituitary–adrenal axis and the stress response. In: McEwen BS (ed). Handbook of Physiology: Coping with the Environment. Oxford University Press: New York. pp 237–270.
Walker CD, Dallman MF (1993). Neonatal facilitation of stress-induced adrenocorticotropin secretion by prior stress: evidence for increased central drive to the pituitary. Endocrinology 132: 1101–1107.
Walker CD, Scribner KA, Cascio CS, Dallman MF (1991). The pituitary–adrenocortical system of neonatal rats is responsive to stress throughout development in a time-dependent and stressor-specific fashion. Endocrinology 128: 1385–1395.
Wong M-L, Licinio J, Pasternak KI, Gold PW (1994). Localization of corticotropin-releasing hormone (CRH) receptor mRNA in adult rat brain by in situ hybridization histochemistry. Endocrinology 135: 2275–2278.
Acknowledgements
This research was supported by NIH Grant MH62099 to JFL, and by NIH Grant HD/DK37431 and a NARSAD Award to DMV. We thank Ms Amy Steffek and Mr Carl Flink for their help with data collection, data analysis, and assistance in the preparation of the photomicrographs.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Vázquez, D., Eskandari, R., Phelka, A. et al. Impact of Maternal Deprivation on Brain Corticotropin-Releasing Hormone Circuits: Prevention of CRH Receptor-2 mRNA Changes by Desipramine Treatment. Neuropsychopharmacol 28, 898–909 (2003). https://doi.org/10.1038/sj.npp.1300126
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.npp.1300126