Abstract
Neuronal cyclin-dependent kinase-5 (Cdk5) and its neuron-specific activator p35 play a major role in regulating the cytoskeleton dynamics. Since opioid addiction was associated with hyperphosphorylation of neurofilament (NF) in postmortem human brains, this study was undertaken to assess the status of the cdk5/p35 complex and its relation with NF-H phosphorylation in brains of chronic opioid abusers. Decreased immunodensities of cdk5 (18%) and p35 (26–44%) were found in the prefrontal cortex of opioid addicts compared with matched controls. In the same brains, the densities of p25 (a truncated neurotoxic form of p35), phosphatase PP2Ac and μ-calpain were found unaltered. Acute treatment of rats with morphine (30 mg/kg, 2 h) increased the density of cdk5 (35%), but not that of p35, in the cerebral cortex. In contrast, chronic morphine (10–100 mg/kg for 5 days) induced marked decreases in cdk5 (40%) and p35 (47%) in rat brain. In brains of opioid addicts, the density of phosphorylated NF-H was increased (43%) as well as the ratio of phosphorylated to nonphosphorylated NF-H forms (two-fold). In these brains, phosphorylated NF-H significantly correlated with p35 (r=0.58) but not with cdk5 (r=0.03). The results suggest that opiate addiction is associated with downregulation of cdk5/p35 levels in the brain. This downregulation and the aberrant hyperphosphorylation of NF-H proteins might have important consequences in the development of neural plasticity associated with opiate addiction in humans.
Similar content being viewed by others
INTRODUCTION
Neuronal cyclin-dependent kinase-5 (Cdk5) is an atypical member of the cyclin-dependent kinase family as its ectopic expression does not induce progression through the cell cycle (Tang and Wang, 1996; Dhavan and Tsai, 2001). Cdk5 is widely distributed in mammalian tissues, but the highest density is detected in the brain (Hellmich et al, 1992). However, monomeric cdk5 does not display enzymatic activity and its activation requires the association with specific noncyclin regulatory proteins, named p35 (neuronal cdk5 activator) and the isoform p39 (Lew et al, 1994; Tsai et al, 1994; Dhavan and Tsai, 2001). The histological distributions of p35 and p39 are similar to that of cdk5, with highest levels in postmitotic neurons and none occurring in glial cells (Zheng et al, 1998). Cdk5 functions in regulating the cytoarchitecture of the CNS. Furthermore, recent evidence also suggests that cdk5 regulates cytoskeletal dynamics and various aspects of synaptic function (Tang and Wang, 1996; Dhavan and Tsai, 2001). Moreover, several studies have implicated dysregulation of cdk5/p35 in the pathophysiology of various neurodegenerative disorders such as Alzheimer's disease, Parkinson's disease, and amyotrophic lateral schlerosis (Nakamura et al, 1997; Dhavan and Tsai, 2001; Maccioni et al, 2001).
During the last few years the neurobiology of drug addiction has focused on the molecular and cellular mechanisms associated with the regulation of synaptic structures, neuronal plasticity, and other adaptive changes in the brain (Nestler, 2001). In this context, chronic treatment of rats with cocaine (which acts as an indirect agonist at D1-dopamine receptors by inhibiting dopamine transporters) has been shown to result in upregulation of cdk5 and of its neuronal activator p35 (mediated by the transcription factor ΔFosB) within the nucleus accumbens and related striatal regions (caudate-putamen) (Bibb et al, 2001). This study indicated that the cdk5/p35 kinase complex is a downstream target of chronic cocaine exposure and therefore of possible relevance in cocaine addiction. In contrast, nothing is known about the regulation of cdk5/p35 by opiate drugs in the brain.
In a previous study (Ferrer-Alcón et al, 2000), opioid addiction in humans was associated with hyperphosphorylation of neurofilament (NF) proteins (NF-H and NF-M subunits), suggesting the induction of changes in neuronal plasticity by chronic opiates. In this context, cdk5 has been shown to phosphorylate NF-H proteins in vitro (including human NF) (Pant et al, 1997; Grant et al, 2001). Other studies also indicate that cdk5/p35 is a major kinase complex regulating the phosphorylation state of these cytoskeletal proteins (Sun et al, 1996; Qi et al, 1998). In turn, protein phosphatase 2Ac (PP2Ac) appears to be the most effective phosphatase in the nervous tissue in dephosphorylating the sites in NF-H known to be phosphorylated by cdk5 (Veeranna et al, 1995). It therefore appears that cdk5/p35 and PP2Ac may mutually regulate the phosphorylation state of NF in neurons (Grant et al, 2001).
Since the potential relevance of aberrant NF hyperphosphorylation in human opioid addiction (Ferrer-Alcón et al, 2000), this study was undertaken to assess mainly the status of the regulatory cdk5/p35 kinase complex and phosphatase PP2Ac and their relations with NF-H phosphorylation in postmortem brains from chronic opioid abusers. A preliminary account of part of this study was presented in Basel at the 31st Annual USGEB/USSBE Meeting (Ferrer-Alcón et al, 1999).
SUBJECTS AND METHODS
Human Brain Samples
Specimens of the right frontal cortex (Brodmann area 9) were collected from 21 well-defined opioid abusers who had died of an opiate overdose, and were immediately stored at −80°C. Drug screening (Table 1) and the determination of a long-term history of opioid addiction were made as described previously (Ferrer-Alcón et al, 2000; Meana et al, 2000). For comparison, in blood specimens of heroin-related deaths, a concentration of 0.22 μg/ml morphine has been found to be lethal (Goldberger et al, 1994), and generally a concentration of over 0.4 μg/ml methadone is sufficient to cause death in subjects tolerant to opioids (Merril et al, 1996). In most opioid addicts, the detection in hair samples of 6-monoacetylmorphine (range 0.2–5.2 ng/mg, n=9) or methadone (range 0.4–14.2 ng/mg, n=7) indicated a long-term abuse of opiates in these subjects (at least 6–12 months before death) (see also Tagliaro et al, 1998; McLeman et al, 2000). Ethanol was found in blood samples from eight opioid addicts (0.56±0.28 g/l), but evidence that these subjects were not ethanol-dependent was obtained. Other drugs detected in blood (eg weak concentrations of cocaine) in some opioid addicts (Table 1) did not appear to alter the status of target proteins in brains of opioid addicts (see Results) (Ferrer-Alcón et al, 2000). Control specimens were obtained at autopsy from potential healthy subjects dying in the same period and who met specific criteria as described previously (Meana et al, 2000) (Table 2). Ethanol was found in blood samples from seven control subjects (0.54±0.31 g/l), but evidence that these subjects were not ethanol-dependent was also obtained. The definitive control group consisted of specimens from 17 subjects with an age at death and a postmortem day (PMD) that did not differ between opioid addicts (Table 1) and controls (Table 2). Tissue storage (time from freezing to immunoblot assay) did not differ between control subjects (mean±SEM, 10±2 months; range, 3–17 months) and opioid addicts (10±2 months; range, 3–16 months). The influence of PMD (range, 3–102 h) and age (range, 16–94 years) on target proteins in the brain was assessed in a similar group of control subjects. This study was approved by the research and ethical review board of the Department of Psychiatry, University of Geneva.
Opiate Drug Treatment of Rats and Brain Samples
Male Sprague–Dawley rats (250–300 g) were used. The animals were housed under controlled environmental conditions (22°C, 70% humidity, and 12-h light/dark cycle) with free access to food and water. For the acute treatments, the rats received a single intraperitoneal (i.p.) injection of morphine (30 mg/kg for 2 h) or naloxone (10 mg/kg for 2 h). For the chronic treatment with morphine, the rats were injected i.p. three times daily during 5 days with increasing doses of the opiate (10–100 mg/kg) (Ventayol et al, 1997), and the animals were killed 2 h after the last dose. Control rats received 0.9% saline vehicle (1 ml/kg i.p. for 2 h) at the indicated treatment times. The rats were killed by decapitation and specimens of cerebral cortex were dissected and stored at −80°C until use. These experiments in rats were performed according to the guidelines of the University of the Balearic Islands, Spain.
Immunoblot Assays and Quantitation of Target Protein Contents
Preparation of cerebral membranes/supernatant (total homogenate or proteins extracted in Triton X-100 containing various protease inhibitors) and quantitation of the target proteins (Western blotting) were assessed as described previously (García-Sevilla et al, 1997; Ventayol et al, 1997; Ferrer-Alcón et al, 2000). In some experiments, specific sodium dodecyl sulfate-polyacrylamide gel electrophoresis SDS-PAGE with large gels (16 × 20 cm2) were performed to assess the effect of PMD on p35 protein immunoreactivity in a large number (n=20) of subjects (linear protein decay vs a monoexponential decay model, see Figure 1c) (Sastre and García-Sevilla, 1994). These gels were also stained with Coomassie brilliant blue dye to assess the uniformity of total protein applied to each lane (data not shown) (Ferrer-Alcón et al, 2000).
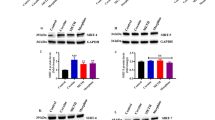
Representative immunoblots for the effect of postmortem delay (PMD: 3–102 h) on the immunoreactivity of (a) cdk5 and (b) activator p35 and truncated p25 protein (detected with antibody anti-p35 C-19) in the human brain (14 healthy subjects: 12 men, 2 women; age: 18–63 years; all subjects run in the same gel). The experiments were repeated twice with very similar results. The molecular masses of cdk5 (35 kDa), p35 (35 kDa), and p25 (25 kDa) were estimated from referenced standards. (c/d) Correlation analyses between PMD and target protein contents (linear and monoexponential decay models). The vertical axes (immunodensities) are expressed as integrated optical densities (IODs).
The following primary antibodies were used: anti-cdk5 (Ab-1, clone DC-17, Lot 129401–4, 1 : 10 000 dilution, Oncogene Research Products, Cambridge, MA, USA), anti-p35 (against the amino terminus, N-20, Lot C028, 1 : 1000 dilution, and against the carboxy terminus, C-19, Lot D270, 1 : 3000 dilution, Santa Cruz Biotechnology, CA, USA; antibody C-19, but not N-20, also recognizes protein p25, a proteolytic product of p35), anti-PP2Ac catalytic α (clone 46, Lot 2, 1 : 20 000 dilution, Transduction Laboratories, Lexington, KY, USA), and anti-μ-calpain 1 (C-20, Lot B180, 1000 dilution, Santa Cruz Biotechnology). For the different forms of NF-H proteins, the following monoclonal antibodies were used: phosphorylated NF-H (anti-NF 200 kDa, SMI-31, batch 13, 1 : 5000 dilution, Sternberger Monoclonals, Baltimore, MD, USA) and nonphosphorylated NF-H (anti-NF 200 kDa, SMI-32, batch 11, 1 : 1000 dilution, Sternberger Monoclonals). α-Internexin, a 64 kDa neuronal filament closely related to NF-L, was quantitated (rabbit anti-α-internexin, 1 : 1000 dilution, Chemicon International Inc., Temecula, CA, USA) as a negative control (Ferrer-Alcón et al, 2000).
The amount of a target protein in the frontal cortex (40 μg in duplicate) of one or two opioid addicts was compared with that of a control subject matched for sex, age (±8 years), and PMD (±12 h), and these subjects were run together on the same gel. This quantification procedure was assessed at least three times in different gels. Finally, percent changes in immunoreactivity with respect to control samples (100%) were calculated for each opioid addict in each gel, and the mean value of the different gels was used as a final estimate. The relative degree of phosphorylation of NF-H in each brain of opioid addicts was expressed as the ratio of SMI-31/SMI-32 immunoreactivity (% of matched controls) (Ferrer-Alcón et al, 2000). Similarly, the content of a target protein in the prefrontal cortex of rats treated with opiate drugs was compared in the same gel with that of control rats, which received saline solution. The quantification procedure was assessed three times in different gels. Finally, percent changes in immunoreactivity with respect to control saline samples (100%) were calculated for each rat treated with morphine or naloxone in the various gels and the mean value was used as a final estimate. Owing to the use of Triton X-100, only the soluble subpopulation of cdk5/p35 was quantitated in the rat brain.
Data Analysis
All series of data were analyzed with the program GraphPad Prism™, version 2.0, and all of them followed Gaussian distributions (Kolmogorov–Smirnov normality test). Results are expressed as mean values±standard error of the mean (SEM). The one-sample t-test or Student's t-test for unpaired values (human data), and one-way ANOVA followed by Scheffé's multiple comparison test (rat data) were used for the statistical evaluations. Pearson correlation coefficients were calculated to test for possible association between variables. The level of significance was chosen as p=0.05.
Drugs and Materials
Morphine HCl was from Unión-Químico-Farmacéutica S.A.E. (Madrid, Spain) and naloxone HCl was from Endo Laboratories (Garden City, NY, USA). Other materials and chemicals were from Amersham International (UK), Sigma Chemie or Fluka Chemie (Buchs, Switzerland).
RESULTS
Effects of PMD and Age on cdk5, p35, and Associated Proteins in Human Brain
In initial studies, the influence of PMD and age on cdk5 and p35 protein immunoreactivity was assessed to determine the pattern of degradation of this complex and the effect of aging. It should be noted that there was no difference in these parameters between the control (PMD: 31±6 h; age: 32±2 years) and opioid addict (PMD: 34±4 h; age: 30±2 years) groups. In brains (prefrontal cortex) of control subjects, the PMD (range 3–102 h) did not alter the immunodensity of cdk5 (r=0.06, n=14, p=0.83) (Figure 1a). In the same brains, however, the immunodensity of p35 (the activator of cdk5) declined with the length of PMD (range 3–102 h; r=−0.74, n=14, p=0.0039) (Figure 1b, c). The decline of p35 protein was rapid and reached a maximum at 24–26 h of PMD and then further declined slowly to an almost disappearance of the immunoreactive band at 86–102 h of PMD (Figure 1b, c), which suggested that the degradation of p35 does not follow the usually observed linear decay model for most brain proteins. To assess for other decay models, the experiment was repeated by using a larger number of brains. Thus, the decline of p35 immunodensity with PMD (3–102 h) also fit well to a monoexponential decay model (r=−0.87, n=20, p=0.0018) in which the rate of protein degradation is not linear but proportional to the protein density at any time (t1/2=19.7 h), and this decay model was better (p<0.01) than the linear model (Figure 1c inset). In contrast, the immunodensity of p25 (a proteolytic product of p35) also declined with the length of PMD (3–102 h) but the pattern of protein degradation was clearly linear (r=−0.93, n=14, p<0.0001) (Figure 1d). In these brains of healthy subjects, no relation between the immunodensities of p35 and p25 proteins was observed for the effect of PMD (ie possible degradation of p35 to p25; note that PMD of 3–22 h were associated with marked decreases in p35 and no change in p25 in the same brains) (Figure 1b–d). On the other hand, PMD (3–102 h) did not alter the immunodensity of μ-calpain (r=0.02, n=14, p=0.94) and PP2Ac (r=−0.22, n=14, p=0.36) in human brain (data not shown).
In brains (prefrontal cortex) of control subjects, there were weak correlations between the age of the subject at death (range: 16–87 years; PMD⩽16 h) and the immunodensities of cdk5 (r=−0.60, n=14, p=0.04), p35 (r=−0.51, n=14, p=0.08), p25 (r=−0.35, n=14, p=0.24), μ-calpain (r=0.24, n=14, p=0.41), and PP2Ac (r=−0.25, n=14, p=0.34), and although the relations were not significant, except for cdk5, the general tendency for these proteins was for a decrease with aging (data not shown). Since opiate addicts were matched with control subjects for PMD and age, it is unlikely that these variables may have influenced the current results in opioid addicts (see below).
Density of cdk5, p35, and Associated Proteins in Brains of Opioid Addicts
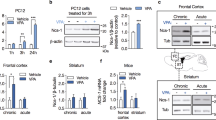
The immunodensity of cdk5 was decreased (18±4%, n=21, t=4.51, p<0.001) in the prefrontal cortex of opioid addicts compared with sex-, age-, and PMD-matched controls (Figure 2a and Table 1 for individual values). In the same brains of opioid addicts, the immunodensity of p35 (the neuron-specific cdk5 activator), quantitated with two different antibodies, also was decreased (antibody N-20: 44±8% decrease, n=21, t=5.16, p<0.001; antibody C-19: 26±9% decrease, n=21, t=2.97, p<0.01) (Figure 2b and Table 1 for individual values). It is important to note that the reduced density of p35 in brains of opioid addicts did not correlate inversely with the length of PMD (r=0.08 for antibody N-20; r=0.37, p=0.10 for antibody C-19; range of PMD: 13–73 h).
Immunodensities of (a) cdk5, PP2Ac, and phosphorylated NF-H (P+NF-H) and of (b) activator p35 (antibodies C-19 and N-20), truncated p25 protein (antibody C-19), and μ-calpain in the prefrontal cortex (Brodmann area 9) of opioid addicts (n=21) as mean±SEM (bars) percentages of the contents in matched controls. *p<0.01, **p<0.001 when compared with matched controls (one-sample t-test). (c) Ratios of p35 to p25 (antibody C-19) immunoreactivity in the prefrontal cortex of opioid addicts and control subjects. Each circle is the ratio p35/p25 (IOD immunoreactive values) for a given subject. The line represents the mean value of each group (see the main text for further details).
In brains of opioid addicts, the immunodensity of p25 (a proteolytic product of p35) did not differ significantly from that in matched controls (8±5% decrease, n=21, t=1.50, p>0.05) (Figure 2b and Table 1 for individual values). In these brains the ratio p35/p25 was significantly lower than that in control subjects (controls: 0.61±0.05, n=15; addicts: 0.46±0.04, n=21, p=0.026) (Figure 2c), further indicating that the content of p35 in human brain is decreased in opioid addiction. Moreover, the immunodensity of p35 correlated with that of cdk5 (r=0.42, n=21, p=0.05) in the same brains of opioid addicts. The immunodensities of PP2Ac (95±4%, n=21, p>0.05) and μ-calpain (108±8%, n=21, p>0.05) were found unchanged in the same brains of opioid addicts (Figures 2a, b). In these brains, the immunodensity of α-internexin, quantitated as a negative control, was not significantly altered (99±6%, n=21, p>0.05) compared with matched controls (data not shown).
In brains of drug-free opioid addicts (no drugs other than opiates and ethanol detected), similar decreases in the immunodensities of cdk5, p35, and ratio p35/p25 were obtained (cdk5: 14±4% decrease, n=14, t=3.27, p<0.005; p35 with antibody N-20: 42±11% decrease, n=14, t=3.91, p<0.005; p35 with antibody C-19: 23±11% decrease, n=14, t=2.13, p=0.05; ratio p35/p25: 0.42±0.04, n=14, p<0.05). Variables such as heroin or methadone overdose, plasma concentrations of opiates and presence of ethanol or other drugs in brain samples did not significantly alter the opiate-induced changes in the cdk5/p35 complex in brains of opioid addicts (data not shown).
Density of Phosphorylated NF-H Proteins in Brains of Opioid Addicts: Relation to the cdk5/p35 Complex
In a previous study (Ferrer-Alcón et al, 2000), increased immunodensities of phosphorylated NF-H and NF-M were demonstrated in a reduced series (n=12) of postmortem brains from opioid addicts. Since the cdk5/p35 complex and phosphatase PP2Ac appear to be major enzymes regulating the state of NF-H phosphorylation, the phosphorylated and nonphosphorylated forms of NF-H were measured in the current larger series of brains of opioid addicts (n=21) in order to assess their possible relation with the alterations of the cdk5/p35 complex.
The immunodensity of phosphorylated NF-H (antibody SMI-31) was markedly increased (43±13%, n=21, t=3.34, p<0.005) in the prefrontal cortex of opioid addicts compared with sex-, age-, and PMD-matched controls (Figure 2a). In the same brains of opioid addicts, and also as expected (Ferrer-Alcón et al, 2000), the density of nonphosphorylated NF-H (antibody SMI-32) was decreased (48±6%, n=21, t=7.47, p<0.0001) (data not shown). In these brains the relative degree of phosphorylation of NF-H (SMI-31/SMI-32: 3.50±0.50, n=21) was very similar to that quantitated previously (Ferrer-Alcón et al, 2000) (opioid addicts, SMI-31/SMI-32: 3.40±0.52, n=12; control subjects, SMI-31/SMI-32: 1.60±0.47, n=9, p<0.02), clearly indicating that a greater proportion (about two-fold) of certain NF-H epitopes are in the phosphorylated form in brains of opioid addicts. In the same brains, the immunodensity of PP2Ac was not significantly altered (5±4% decrease, p>0.05). In brains of drug-free opioid addicts (no drugs other than opiates and ethanol detected), similar changes in the immunodensities of NF-H proteins were obtained (phosphorylated NF-H: 49±18% increase, n=14, t=2.81, p<0.02; nonphosphorylated NF-H: 43±9% decrease, n=14, t=4.79, p<0.001; ratio SMI-31/SMI-32: 3.46±0.65). Variables such as PMD, age of the subjects, heroin or methadone overdose, plasma concentrations of opiates and presence of ethanol or other drugs in brain samples did not alter significantly the opiate-induced changes of NF-H proteins in brains of opioid addicts (data not shown).
In brains (prefrontal cortex) of opioid addicts, there was a positive and significant correlation (r=0.58, n=42, p<0.0001) between the immunodensity of p35 (antibodies C-19 and N-20) and that of phosphorylated NF-H (Figure 3a). In marked contrast, the immunodensity of cdk5 did not correlate (r=0.03, n=21) with that of phosphorylated NF-H in the same brains (Figure 3b). However, a multiple regression analysis between the immunodensity of phosphorylated NF-H (dependent variable) and those of p35 and cdk5 (independent variables) in the same brain samples of opioid addicts also resulted in a positive and significant correlation (r=0.57, n=21, p=0.026). In the same brains, the abundance of PP2Ac did not correlate significantly with that of phosphorylated NF-H (r=0.29, n=21, p=0.195).
(a) Scatterplot depicting a significant correlation between the immunodensities of activator p35 (black circles, antibody C-19; white circles, antibody N-20) and phosphorylated NF-H (P+NF-H) in the prefrontal cortex (Brodmann area 9) of opioid addicts. Each circle represents a different opioid addict (for C-19 or N-20 antibody) and the solid line is the regression of the correlation. For each p35 antibody the correlation also was positive and significant (for antibody C-19: r=0.54, n=21, p=0.012; for antibody N-20: r=0.67, n=21, p=0.001). (b) Scatterplot depicting the lack of correlation between the immunodensities of cdk5 and P+NF-H in the prefrontal cortex of the same opioid addicts.
Acute and Chronic Effects of Morphine and Naloxone on cdk5 and p35 in Rat Brain
Since the downregulation of the cdk5/p35 complex in brains of opioid addicts could be related to both the chronic effect of the opiates and the final acute effect of the deadly opiate overdose, the influence of the opioid system on this regulatory complex was investigated in the rat brain. Acute morphine (30 mg/kg, i.p. for 2 h) increased the immunodensity of cdk5 (35±6%, n=3, p<0.05), but not that of its activator p35, in the cerebral cortex (Figure 4a, b). In contrast, chronic treatment (5 days) with morphine induced similar decreases in the immunodensities of cdk5 (40±13%, n=6, p<0.05) and p35 (47±5%, n=6, p<0.05) in brain (Figure 4a, b). Acute and chronic morphine did not modify significantly (ANOVA, F(3,10)=1.12, p=0.39) the immunodensity of p25 protein in brain (Figure 4b). The acute treatment with naloxone (10 mg/kg, i.p. for 2 h), a nonselective opioid receptor antagonist, showed a clear tendency to decrease the immunodensity of cdk5 (26±6%, p=0.07), but not that of p35, in the rat cerebral cortex (Figure 4a, b).
Effects of acute (30 mg/kg, i.p., 2 h) and chronic morphine (10–100 mg/kg for 5 days) treatments and of acute naloxone (10 mg/kg, i.p., 2 h) on the immunodensities of cdk5 and activator p35 in rat brain (cerebral cortex). (a) Columns are means±SEM of 3–6 experiments per group with an animal per experiment, and expressed as percentage of saline-treated rats (n=5). *p<0.05 when compared with the saline group (ANOVA followed by Scheffé's test). (b) Representative immunoblots for the effect of opiate drugs on cdk5, p35, and p25 in the cerebral cortex. The molecular masses of cdk5 (35 kDa), p35 (35 kDa), and p25 (25 kDa) were estimated from referenced standards. The rat brain samples were first tested with the polyclonal antibody C-19 (p35/p25) and then (same blotted gel) with the monoclonal antibody Ab-1 (cdk5), followed in each case by protein immunodetection with the appropriate peroxidase-linked secondary antibody. Although the molecular mass and physical appearance of the bands labeled cdk5 and p35 are similar, this is most likely because of migration artifacts in the gel affecting all proteins at these locations since we employed different species of primary and secondary antibodies for these blots. Note that p35 was immunodetected as a doublet with a less intense band of higher molecular mass (probably an unknown related peptide), which was similarly modulated by opiate drugs. A similar chronic morphine treatment in mice also decreased the immunodensity of cdk5 and p35 (separately blotted gels) in brain (data not shown).
DISCUSSION
The main results of this study demonstrate that opioid addiction in humans and in rats is associated with downregulation of neuronal cdk5/p35, a regulatory enzymatic complex that is involved in the regulation of the state of phosphorylation of cytoskeletal NF-H proteins.
In brains of healthy subjects, the immunodensities of p35 (kinase activator) and p25 (proteolytic product) declined with the length of PMD, as is the general case for most brain proteins (Fountoulakis et al, 2001). In contrast, the immunodensities of cdk5, μ-calpain, and phosphatase PP2Ac were not affected by the PMD (up to 102 h). Interestingly, the degradation of p35 (in contrast to that of p25) was very rapid and it followed a monoexponential decay with a half-life of 19.7 h. In vitro studies have also shown that the degradation of p35 by the ubiquitin-proteasome pathway is rapid and that the truncated form p25 is a stable protein (Patrick et al, 1998). In the human brain, the lack of an inverse relation between p35 and p25 proteins (possible postmortem conversion) indicated that p35 is not cleaved to p25 during its postmortem proteolytic degradation. Recently, it has been suggested that p25 accumulates in neurons in brains of patients with Alzheimer's disease (20–40-fold compared to p35), leading to an increased cdk5 activity, hyperphosphorylation of protein tau as well as NF-H proteins, and finally to neurodegeneration (Patrick et al, 1999). The mechanism of conversion of p35 to p25 involves the enzyme μ-calpain, which directly cleaves p35 to release the fragment p25 (Lee et al, 2000; Kusakawa et al, 2000). In this context, it is of interest to note that in brains of opioid addicts the levels of p25 and μ-calpain were not different from those in control subjects, indicating that opiate drugs do not alter the mechanisms of in vivo conversion of p35 to p25.
In opioid addicts the immunodensity of cdk5 and that of its activator p35 were decreased in the prefrontal cortex. Since the abundance of p25 in these brains remained unchanged, the ratio p35/p25 in opioid addicts was lower than that in control subjects, further demonstrating the downregulation of p35 in opioid addiction. The observed downregulation of the cdk5/p35 complex could be related to the deadly overdose (heroin or methadone), which would mimic an acute effect of the opiate. However, acute morphine in rats was associated with upregulation of cdk5 in the cerebral cortex. In contrast, chronic morphine decreased the levels of cdk5 and p35 in the rat brain. It therefore appears that, in spite of the possible stimulating effect of the deadly opiate overdose on cdk5, the reduced expression of the cdk5/p35 complex in brains of opioid addicts is the net result of a chronic opiate effect. Cdk5/p35 is subject to rapid turnover and it has been suggested that the rate of p35 proteosomal degradation is controlled through autophosphorylation by cdk5 (Patrick et al, 1998). Sustained stimulation of the μ-opioid receptor system may result in downregulation of the cdk5/p35 complex via this mechanism. Since acute morphine increased and acute naloxone showed a clear tendency to decrease the expression of cdk5 in rat brain, the existence of a tonic regulation on this kinase induced by opioid peptides (ie endomorphines), through opioid receptors, cannot be discarded.
Recently, chronic treatment of rats with the psychostimulant cocaine has been shown to result in upregulation of cdk5 and p35 in brain (nucleus accumbens, caudate-putamen) (Bibb et al, 2001). The chronic effects of morphine (current results) and cocaine on this common downstream target are most interesting, because they suggest an opposite regulation of the cdk5/p35 complex upon the sustained inhibition (μ-opioid receptors) or activation (D1-dopamine receptors) of the adenylyl cyclase/cAMP/protein kinase A pathway, which might be of relevance in opioid and cocaine addiction. However, the chronic effects of cocaine on the cdk5/p35 complex (upregulation) were mediated through the induction of the transcription factor ΔFosB (Bibb et al, 2001), and the accumulation of ΔFosB in brain also has been observed after chronic morphine administration (Nye and Nestler, 1996; Nestler et al, 2001). Owing to the multiple and complex opioid receptor signaling (Law et al, 2000), different molecular mechanisms may account for the downregulation of the cdk5/p35 complex induced by chronic morphine exposure.
One relevant target of the cdk5/p35 complex, working in association with phosphatase PP2Ac, is to regulate the state of phosphorylation of NF proteins in neurons (review in Grant et al, 2001). In line with previous findings (Ferrer-Alcón et al, 2000), the immunodensity of phosphorylated NF-H, but not that of PP2Ac, was increased in the prefrontal cortex of opioid addicts, suggesting that the hyperphosphorylation of NF-H is not the result of a major defect in the dephosphorylation process of these cytoskeletal proteins. On the other hand, the abundance of cdk5 was decreased in the same brains, which could suggest that this kinase is not directly responsible for the observed effects of opiate drugs in NF-H phosphorylation. In fact, no correlation was found between the levels of cdk5 and phosphorylated NF-H in the same brains of opioid addicts (Figure 3). This apparent discrepancy deserves an explanation. In rat brain acute morphine increases both the abundance of cdk5 and the phosphorylation of NF-H (93%) (Jaquet et al, 2001), whereas chronic opiate treatment (tolerant state) was associated with downregulation of cdk5 and also with the induction of tolerance to the acute effect of morphine on the phosphorylation of NF-H (Jaquet et al, 2001). Therefore, these observations in the rat brain suggest that the hyperphosphorylated NF-H proteins in brains of opioid addicts (with concomitant downregulation of cdk5) can be related to both the chronic effect of the opiate and the final acute effect of the deadly opiate overdose. In this context, it is interesting to note that also the abundance of p35 was decreased in brains of opioid addicts but, in contrast to cdk5, a positive correlation was found between the immunodensities of p35 and phosphorylated NF-H in each brain, clearly suggesting a close relation between the kinase activator and the phosphorylation of the target protein (ie low levels of p35 were associated with reduced or unaltered NF-H phosphorylation whereas higher p35 levels were associated with a more pronounced NF-H phosphorylation; Figure 3). This correlation might also indicate, as first suggested in vitro (Qi et al, 1998), that p35 not only activates cdk5 but also serves as an anchoring protein to localize the kinase to its protein substrates, which would provide a molecular mechanism for the colocalization of NF and the cdk5/p35 complex (Qi et al, 1998; Veeranna et al, 2000).
The downregulation of the cdk5/p35 complex and the aberrant hyperphosphorylation of NF-H proteins observed in postmortem brains of opioid addicts might have important consequences in the long-term development of various forms of neural plasticity associated with opiate addiction in humans (Ferrer-Alcón et al, 2000; Nestler, 2001).
References
Bibb JA, Chen J, Taylor JR, Svenningsson P, Nishi A, Snyder GL (2001). Effects of chronic exposure to cocaine are regulated by the neuronal protein Cdk5. Nature 410: 376–380.
Dhavan R, Tsai L-H (2001). A decade of CDK5. Nature Rev/Mol Cell Biol 2: 749–759.
Ferrer-Alcón F, García-Sevilla JA, La Harpe R, Walzer C, Guimón J (1999). Regulation of p35/cyclin-dependent kinase 5 (p35/cdk-5) complex in brains of opioid addicts. S07-10, p 49.
Ferrer-Alcón F, García-Sevilla JA, Jaquet Ph, La Harpe R, Riederer BM, Walzer C et al (2000). Regulation of nonphosphorylated and phosphorylated forms of neurofilament proteins in the prefrontal cortex of human opioid addicts. J Neurosci Res 61: 338–349.
Fountoulakis M, Hardmeier R, Höger H, Lubec G (2001). Postmortem changes in the level of brain proteins. Exp Neurol 167: 86–94.
García-Sevilla JA, Ventayol P, Busquets X, La Harpe R, Walzer C, Guimón J (1997). Marked decrease of immunolabelled 68 kDa neurofilament (NF-L) proteins in brains of opiate addicts. NeuroReport 8: 1561–1570.
Goldberger BA, Cone EJ, Grant T, Caplan YH, Levine BS, Smialek JE (1994). Disposition of heroin and its metabolites in heroin-related deaths. J Anal Toxicol 18: 22–28.
Grant P, Sharma P, Pant HC (2001). Cyclin-dependent protein kinase 5 (CDK5) and the regulation of neurofilament metabolism. Eur J Biochem 268: 1534–1546.
Hellmich MR, Pant HC, Wada E, Battey JF (1992). Neuronal cdc2-like kinase: a CDC2-related protein kinase with predominantly neuronal expression. Proc Natl Acad Sci USA 89: 10867–10871.
Jaquet PhE, Ferrer-Alcón M, Ventayol P, Guimón J, García-Sevilla JA (2001). Acute and chronic effects of morphine and naloxone on the phosphorylation of neurofilament-H proteins in the rat brain. Neurosci Lett 304: 37–40.
Kusakawa G-I, Saito T, Onuki R, Ishiguro K, Kishimoto T, Hisanaga S-I (2000). Calpain-dependent proteolytic cleavage of the p35 cyclin-dependent kinase 5 activator to p25. J Biol Chem 275: 17166–17172.
Law P-Y, Wong YH, Loh HH (2000). Molecular mechanisms and regulation of opioid receptor signaling. Annu Rev Pharmacol 40: 389–430.
Lee M-S, Kwon YT, Li M, Peng J, Friedlander RM, Tsai L-H (2000). Neurotoxicity induces cleavage of p35 to p25 by calpain. Nature 405: 360–364.
Lew J, Huang Q-Q, Qi Z, Winkfein RJ, Aebersold R, Hunt T et al (1994). A brain-specific activator of cyclin-dependent kinase 5. Nature 371: 423–426.
Maccioni RB, Otth C, Concha II, Muñoz JP (2001). The protein kinase Cdk5. Structural aspects, roles in neurogenesis and involvement in Alzheimer's pathology. Eur J Biochem 268: 1518–1527.
McLeman ER, Warsh JJ, Ang L, Li PP, Kalasinsky KS, Ross BM et al (2000). The human nucleus accumbens is highly susceptible to G protein down-regulation by methamphetamine and heroin. J Neurochem 74: 2120–2126.
Meana JJ, González-Maeso J, García-Sevilla JA, Guimón J (2000). μ -opioid receptor and α2-adrenoceptor agonist stimulation of 35SGTPγS binding to G-proteins in postmortem brains of opioid addicts. Mol Psychiatry 5: 308–315.
Merril J, Garvey T, Rosson C (1996). Methadone concentrations taken as indicating deaths due to overdose need to be reviewed. Br Med J 313: 1481.
Nakamura S, Kawamoto Y, Nakano N, Akiguchi I, Kimura J (1997). p35nck5a and cyclin-dependent kinase 5 colocalize in Lewy bodies of brains with Parkinson's disease. Acta Nueropathol 94: 153–157.
Nestler EJ (2001). Molecular basis of long-term plasticity underlying addiction. Nat Rev/Neurosci 2: 119–128.
Nestler EJ, Barrot M, Self DW (2001). ΔFosB: a sustained molecular switch for addiction. Proc Natl Acad Sci USA 98: 11042–11046.
Nye HE, Nestler EJ (1996). Induction of chronic Fos-related antigens in rat brain by chronic morphine administration. Mol Pharmacol 49: 636–645.
Pant AC, Veeranna GJ, Pant HC, Amin N (1997). Phosphorylation of human high molecular weight neurofilament protein (hNF-H) by neuronal cyclin-dependent kinase 5 (cdk5). Brain Res 765: 259–266.
Patrick GN, Zhou P, Kwon YT, Howley PM, Tsai L-H (1998). p35, the neuronal-specific activator of cyclin-dependent kinase 5 (Cdk5) is degraded by the ubiquitin-proteasome pathway. J Biol Chem 273: 24057–24064.
Patrick GN, Zukerberg L, Nikolic M, de la Monte S, Dikkes P, Tsai L-H (1999). Conversion of p35 to p25 deregulates Cdk5 activity and promotes neurodegeneration. Nature 402: 615–622.
Qi Z, Tang D, Zhu X, Fujita DJ, Wang JH (1998). Association of neurofilament proteins with neuronal Cdk5 activator. J Biol Chem 273: 2329–2335.
Sastre M, García-Sevilla JA (1994). Density of alpha-2A adrenoceptors and Gi proteins in the human brain: ratio of high affinity agonist sites to antagonist sites and effect of age. J Pharmacol Exp Ther 269: 1062–1072.
Sun D, Leung CL, Liem RKH (1996). Phosphorylation of the high molecular weight neurofilament protein (NF-H) by Cdk5 and p35. J Biol Chem 271: 14245–14251.
Tagliaro F, De Battisti Z, Smith FP, Marigo M (1998). Death from heroin overdose: findings from hair analysis. Lancet 351: 1923–1925.
Tang D, Wang JH (1996). Cyclin-dependent kinase 5 (Cdk5) and neuron-specific Cdk5 activators. Prog Cell Cycle Res 2: 205–216.
Tsai L-H, Delalle I, Caviness Jr VS, Chae T, Harlow E (1994). p35 is a neural-specific regulatory subunit of cyclin-dependent kinase 5. Nature 371: 419–423.
Veeranna GJ, Shetty KT, Link WT, Jaffe H, Wang J, Pant HC (1995). Neuronal cyclin-dependent kinase-5 phosphorylation sites in neurofilament protein (NF-H) are dephosphorylated by protein phosphatase 2A. J Neurochem 64: 2681–2690.
Veeranna GJ, Shetty KT, Takahashi M, Grant Ph, Pant HC (2000). Cdk5 and MAPK are associated with complexes of cytoskeletal proteins in rat brain. Mol Brain Res 76: 229–236.
Ventayol P, Busquets X, García-Sevilla JA (1997). Modulation of immunoreactive protein kinase C-α and β isoforms and G proteins by acute and chronic treatments with morphine and other opiate drugs in rat brain. Naunyn-Schmiedeberg's Arch Pharmacol 355: 491–500.
Zheng M, Leung CL, Liem RK (1998). Region-specific expression of cyclin-dependent kinase 5 (Cdk5) and its activators, p35 and p39, in the developing and adult rat central nervous system. J Neurobiol 35: 141–159.
Acknowledgements
This study was supported by Grant 32–57066.99 (JAG-S) from the Fonds National Suisse de la Recherche Scientifique (FNSRS, Bern, Switzerland), and by Grant BFI 2000–0306 (JAG-S) from the Ministerio de Ciencia y Tecnología (MCT, Madrid, Spain). MF-A was supported by a predoctoral fellowship from the FNSRS. JAG-S is a member of the Institut d'Estudis Catalans (Barcelona, Spain).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ferrer-Alcón, M., La Harpe, R., Guimón, J. et al. Downregulation of Neuronal cdk5/p35 in Opioid Addicts and Opiate-Treated Rats: Relation to Neurofilament Phosphorylation. Neuropsychopharmacol 28, 947–955 (2003). https://doi.org/10.1038/sj.npp.1300095
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.npp.1300095
Keywords
This article is cited by
-
5-HT2A receptor- and M1 muscarinic acetylcholine receptor-mediated activation of Gαq/11 in postmortem dorsolateral prefrontal cortex of opiate addicts
Pharmacological Reports (2021)
-
Enhanced Cdk5 Activity and p35 Translocation in the Ventral Striatum of Acute and Chronic Methamphetamine-Treated Rats
Neuropsychopharmacology (2005)
-
Candidate genes, pathways and mechanisms for bipolar (manic–depressive) and related disorders: an expanded convergent functional genomics approach
Molecular Psychiatry (2004)