Abstract
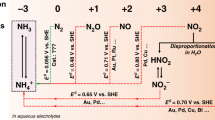
L. ANDRUSSOW (Ber. 59, 458, 1926) has suggested a scheme of reaction for the oxidation of ammonia in which nitroxyl, NOH, is an intermediate product. The scheme put forward by Andrussow, together with many others, were derived by the present writer some years ago when engaged in experimental work on ammonia oxidation, but none of these has been published. It seems desirable, however, to point out that the scheme put forward by Andrussow suffers from the defect that nitroxyl, NOH, if formed as an intermediate product, might be expected to break down to a considerable extent into nitrous oxide, a substance which, so far as the writer is aware, has never been detected among the products of the oxidation of ammonia. A more likely intermediate product would be nitrohydroxylamic acid, which is known to break down with formation of nitric oxide. It is suggested that the oxidation may occur in the following stages, each reaction in which is bimolecular or unimolecular:
Similar content being viewed by others
Article PDF
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
PARTINGTON, J. The Oxidation of Ammonia. Nature 117, 590 (1926). https://doi.org/10.1038/117590c0
Published:
Issue Date:
DOI: https://doi.org/10.1038/117590c0
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.