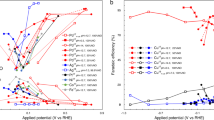
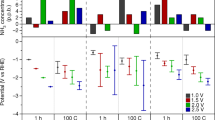
Abstract
Electroreduction of the oxidized forms of nitrogen (NOx) has been studied for decades, with the most recent intense research focusing on the conversion of nitrate to ammonia. Here we discuss how such technology might, or might not, be able to contribute to sustainable ammonia (NH3) production and highlight its conceptual and experimental differences from the electrosynthesis of NH3 from dinitrogen. A generic experimental protocol for NOx-to-NH3 studies is proposed and discussed along with the situations where control experiments with a 15N-labelled nitrogen source may not be required.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Choi, J. et al. Identification and elimination of false positives in electrochemical nitrogen reduction studies. Nat. Commun. 11, 5546 (2020).
Tanaka, H. et al. Unique behaviour of dinitrogen-bridged dimolybdenum complexes bearing pincer ligand towards catalytic formation of ammonia. Nat. Commun. 5, 3737 (2014).
Fichter, F., Girard, P. & Erlenmeyer, H. Elektrolytische Bindung von komprimiertem Stickstoff bei gewöhnlicher Temperatur. HCA 13, 1228–1236 (1930).
Lazouski, N., Chung, M., Williams, K., Gala, M. L. & Manthiram, K. Non-aqueous gas diffusion electrodes for rapid ammonia synthesis from nitrogen and water-splitting-derived hydrogen. Nat. Catal. 3, 463–469 (2020).
Du, H. L. et al. Electroreduction of nitrogen with almost 100% current-to-ammonia efficiency. Nature 609, 722–727 (2022).
Fu, X. et al. Continuous-flow electrosynthesis of ammonia by nitrogen reduction and hydrogen oxidation. Science 379, 707–712 (2023).
van Langevelde, P. H., Katsounaros, I. & Koper, M. T. M. Electrocatalytic nitrate reduction for sustainable ammonia production. Joule 5, 290–294 (2021).
Theerthagiri, J. et al. Electrocatalytic conversion of nitrate waste into ammonia: a review. Environ. Chem. Lett. 20, 2929–2949 (2022).
MacFarlane, D. R. et al. A roadmap to the ammonia economy. Joule 4, 1186–1205 (2020).
International Energy Agency. Ammonia Technology Roadmap: Towards more Sustainable Nitrogen Fertiliser Production (IEA, 2021); https://www.iea.org/reports/ammonia-technology-roadmap
International Renewable Energy Agency. Innovation Outlook: Renewable Ammonia (IRENA, 2022); https://www.irena.org/publications/2022/May/Innovation-Outlook-Renewable-Ammonia
MacFarlane, D., Simonov, A. N., Vu, T., Johnston, S. & Azofra, L. M. Sustainable nitrogen activation—are we there yet? Faraday Discuss. 243, 557–570 (2023).
Hager, T. The Alchemy of Air: a Jewish Genius, a Doomed Tycoon, and the Scientific Discovery that Fed the World but Fueled the Rise of Hitler (Harmony Books, 2008).
Alves, L. et al. A comprehensive review of NOx and N2O mitigation from industrial streams. Renew. Sustain. Energy Rev. 155, 111916 (2022).
Ergas, S. J. & Aponte-Morales, V. in Comprehensive Water Quality and Purification, Vol. 3 (ed. Ahuja, S.) 123–149 (Elsevier, 2014).
International Atomic Energy Agency. Status and Trends in Spent Fuel and Radioactive Waste Management (IAEA, 2022).
US Nuclear Regulatory Commission. Low-Level Waste Disposal Statistics (NRC, 2023); https://www.nrc.gov/waste/llw-disposal/licensing/statistics.html
Anner, N. Upgrading Your Fleet for Future Fuels (MAN Energy Solutions, 2022); https://www.man-es.com/discover/potential-dual-fuel-conversions
International Energy Agency. World Energy Outlook 2022 (IEA, 2022); https://www.iea.org/reports/world-energy-outlook-2022
Lide, D. R. CRC Handbook of Chemistry and Physics, 84th edn (CRC Press, 2004).
Smith, C., Hill, A. K. & Torrente-Murciano, L. Current and future role of Haber-Bosch ammonia in a carbon-free energy landscape. Energy Environ. Sci. 13, 331–344 (2020).
Jupiter Ionics. Global Leaders in Green Ammonia (Jupiter Ionics, 2021); https://jupiterionics.com/
Chen, J. G. et al. Beyond fossil fuel-driven nitrogen transformations. Science 360, eaar6611 (2018).
Sugiyama, K. et al. Ammonia synthesis by means of plasma over MgO catalyst. Plasma Chem. Plasma Process. 6, 179–193 (1986).
Sun, J. et al. A hybrid plasma electrocatalytic process for sustainable ammonia production. Energy Environ. Sci. 14, 865–872 (2021).
PlasmaLeap; https://www.plasmaleap.com
Muzammil, I. et al. Plasma catalyst-integrated system for ammonia production from H2O and N2 at atmospheric pressure. ACS Energy Lett. 6, 3004–3010 (2021).
Wu, A. et al. Direct ammonia synthesis from the air via gliding arc plasma integrated with single atom electrocatalysis. Appl. Catal. B 299, 120667 (2021).
Plieth, W. J. in Encyclopedia of Electrochemistry of the Elements (eds. Bard, A, J. & Lund, H.) Ch. VIII-5, 321–479 (Dekker, 1978).
Rosca, V., Duca, M., de Groot, M. T. & Koper, M. T. M. Nitrogen cycle electrocatalysis. Chem. Rev. 109, 2209–2244 (2009).
Mattarozzi, L. et al. Electrochemical reduction of nitrate and nitrite in alkaline media at CuNi alloy electrodes. Electrochim. Acta 89, 488–496 (2013).
Bui, T. S., Lovell, E. C., Daiyan, R. & Amal, R. Defective metal oxides: lessons from CO2RR and applications in NOxRR. Adv. Mater. 35, 2205814 (2023).
Han, S. et al. Ultralow overpotential nitrate reduction to ammonia via a three-step relay mechanism. Nat. Catal. 6, 402–414 (2023).
Andersen, S. Z. et al. A rigorous electrochemical ammonia synthesis protocol with quantitative isotope measurements. Nature 570, 504–508 (2019).
Hodgetts, R. Y. et al. Refining universal procedures for ammonium quantification via rapid 1H NMR analysis for dinitrogen reduction studies. ACS Energy Lett. 5, 736–741 (2020).
Aouina, N., Cachet, H., Debiemme-chouvy, C. & Tran, T. T. M. Insight into the electroreduction of nitrate ions at a copper electrode, in neutral solution, after determination of their diffusion coefficient by electrochemical impedance spectroscopy. Electrochim. Acta 55, 7341–7345 (2010).
Acknowledgements
We are grateful to the Australian Research Council for financial support (project no. DP200101878 and Future Fellowship FT200100317 to A.N.S.).
Author information
Authors and Affiliations
Contributions
All authors contributed to the preparation and writing of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
D.R.M. and A.N.S. have minority equity ownership, as well as management and consulting roles, in a spin-out company, Jupiter Ionics Pty Ltd, that is scaling up the Li-mediated NH3 electrosynthesis technology.
Peer review
Peer review information
Nature Catalysis thanks the anonymous reviewers for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
John, J., MacFarlane, D.R. & Simonov, A.N. The why and how of NOx electroreduction to ammonia. Nat Catal 6, 1125–1130 (2023). https://doi.org/10.1038/s41929-023-01060-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41929-023-01060-w