Abstract
Experimental characterization of the transition state poses a significant challenge due to its fleeting nature. Negative ion photodetachment offers a unique tool for probing transition states and their vicinity. However, this approach is usually limited to Franck-Condon regions. For example, high-lying Feshbach resonances with an excited HF stretching mode (vHF = 2-4) were recently identified in the transition-state region of the F + NH3 → HF + NH2 reaction through photo-detaching FNH3− anions, but the direct photodetachment failed to observe the lower-lying vHF = 0,1 resonances and bound states due apparently to negligible Franck-Condon factors. Indeed, these weak transitions can be resonantly enhanced via a dipole-bound state (DBS) formed between an electron and the polar FNH3 species. In this study, we unveil a series of Feshbach resonances and bound states along the F + NH3 reaction path via a DBS by combining high-resolution photoelectron spectroscopy with high-level quantum dynamical computations. This study presents an approach for probing the activated complex in a reaction by negative ion photodetachment through a DBS.
Similar content being viewed by others
Introduction
Chemical reactions are always accompanied by the formation and cleaving of chemical bonds1. Right in the midst of this bond rearrangement, the system transforms from the reactant channel to the product channel through an activated complex that is called a transition state2. The transition state is typically located near a saddle point on the potential energy surface (PES) and is a key concept in chemistry for understanding chemical reactivity and kinetics3,4. As a result, an experimental characterization of the transition state has long been a holy grail in chemistry. However, it represents a significant challenge due to its transient nature. The motivation to better understand the transition state has spurred the development of the crossed molecular-beam technique5,6, which has greatly improved our understanding of chemical dynamics7,8,9. In these experiments, the transition state is not directly probed, but its impact on reaction dynamics can be deduced from scattering attributes.
A complementary and more direct experimental method to characterize the transition state and its vicinity is the anion photoelectron spectroscopy10,11,12,13,14,15. Photodetachment of the anion projects its vibrational wavefunction vertically onto the neutral PES by ejecting the electron. If a stable anionic complex exists with a geometry similar to the transition state of the corresponding neutral reaction, the vibrationally resolved photoelectron spectrum contains detailed information on dynamics near the transition state16,17. Their assignment can be revealed by comparison with accurate quantum dynamical computations18,19,20,21. This technique is particularly suited for identifying metastable resonances, which are prevalent in many reactions and capable of impacting the reactivity22. It should be noted that a transition state is not a single geometry but an active complex in a region near the saddle point, often characterized by dynamical resonances22.
Recently, Neumark and coworkers have demonstrated several exquisite experiments using the cryogenic slow electron velocity-map imaging method (Cryo-SEVI) to probe resonances spanning the transition-state region for F + H223, F + CH3OH24, and F + NH325. Cryo-SEVI15,26,27, featuring a very high energy resolution for slow photoelectrons, typically a few cm−1 near the photodetachment threshold, is an excellent tool for resolving complicated vibrational structures. In these experiments, however, the observability of the vibrational structure on the neutral PES is limited to the Franck–Condon region, namely, only those with large overlaps with the anion vibrational wavefunction are detectable. In a recent cryo-SEVI experiment25, for example, Babin et al. observed a series of peaks in the photoelectron spectrum of the FNH3− anion. With the help of quantum dynamics calculations, these peaks were assigned to Feshbach resonances with an excited HF stretching mode (νHF = 2–4) as well as several higher ones straddling the saddle point for the F + NH3 → HF + NH2 reaction. The absence of the lower-lying νHF = 0,1 resonances is presumably due to their negligible Franck–Condon factors.
A polar molecule with a dipole moment >2.5 D can support a dipole-bound state (DBS)28,29, in which the extra electron is loosely bounded at the positively charged end through charge–dipole attraction, with a very low binding energy, typically ~a few tens of meV. The PES of the dipole-bound anion is nearly identical to the neutral PES since the neutral core is only slightly perturbed by the very diffusive DBS electronic wavefunction30,31. The loosely bound electron can serve as a messenger for the dynamic evolution of the neutral transition state32. Once the anion is excited to a DBS with a vibrationally excited neutral core, autodetachment occurs due to the vibronic coupling. This process can potentially enhance the observability of some vibrational states that may be too weak to be detected in direct photodetachment. The existence of a DBS state also enables the pump-probe type experiment to observe ultrafast electronic dynamics, as recently demonstrated by Kim33,34, Verlet35,36,37, and their coworkers.
In this article, we present a joint cryo-SEVI and quantum dynamics study of the F + NH3 → HF + NH2 reaction, in which the low-lying resonances and bound states are detected via the DBS of the FNH3− anion. This work complements the earlier direct photodetachment experiment by Babin et al. 25 and provides a complete mapping of the transition-state region for the F + NH3 reaction, which is a prototype for chemical reactions with a submerged reaction barrier38.
Results
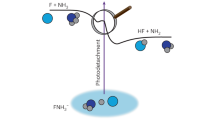
The highly exoergic F + NH3 → HF + NH2 reaction has a submerged barrier flanked by the reactant complex (RC) and product complex (PC) wells. Figure 1 illustrates the schematic representation of how resonances in the transition-state region of the F + NH3 reaction can be probed through the photoelectron detachment of FNH3− anions. The PC resonances with vHF = 2–4 have good Franck–Condon overlaps with the vibrational ground state of the anion, allowing for probing of these states via direct photodetachment. However, the overlaps with vHF = 1 states are quite weak, with roughly one order of magnitude smaller Franck–Condon factors (FCFs) than those of the vHF = 2 states. The overlaps with vHF = 0 states are even smaller. As a result, their signals in direct photodetachment are very weak, as noted in the recent work of Babin et al.25. However, DBS resonances can be photoexcited because they have a much larger cross-section than direct photodetachment, which can then autodetach, as depicted in Fig. 1. Additionally, the observation of these weak signals relies on the production of a strong FNH3− anion beam and the use of high-intensity photodetachment lasers.
The upper bold curve represents the minimum energy path of the F + NH3 reaction, connecting the reactants and products in their ground vibrational states. Three black curves sketched above it are neutral vibrational adiabatic potentials for the excited HF stretching mode (νHF = 1–3), and a red curve is for the dipole-bound state (DBS) of FNH3− anion with νHF = 1. The molecular orbital of the DBS is schematically plotted on a molecular structure next to the curve. Purple and light blue represent their different phases. The lower thick curve represents the anion PES. Structures around these curves are illustrated for the anion and the neutral reactant complex (RC), product complex (PC), and transition state (TS) in gray (H), dark blue (N), and light blue (F). The autodetachment of the DBS provides a bridge to access regions that are difficult to probe using the direct photodetachment, mainly due to the larger geometric difference between the anion and the neutral complex with νHF = 0. Energies shown in parentheses (in cm−1) are zero-point energy corrected and are relative to the reactant asymptote.
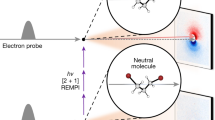
To access the DBS, much longer photon wavelengths than those in the previous cryo-SEVI work (hv > 27,000 cm−1)25 are used. At the lowest energy (hv = 23,340 cm−1), only a single peak (0a) is present. As the photon energy increases, more and more peaks (0b–0j) emerge. A key characteristic of DBS is the vibrational-state-specific enhancement depending on the photon wavelength. As shown in Fig. 2, the intensities of these peaks change dramatically as the photon energy increases, in contrast with the direct photodetachment, where the intensities of peaks are primarily governed by the FCFs and are proportional to (Ek)1/2 for a s-wave Wigner threshold photodetachment39. Here, Ek is the kinetic energy of photoelectrons. This characteristic clearly establishes the involvement of the DBS.
The intensity of peaks labeled 0a–0j depends sensitively on the photon energy due to the photoexcitation of different vibrational states of the DBS. The number 0 in the label represents all peaks observed in this figure assigned to the band vHF = 0, which cannot be observed in the direct photodetachment experiment. Theoretical spectrum convoluted with a 50-cm−1 FWHM Gaussian function (red) is shifted 183 cm−1 higher in energy and plotted at the bottom for comparison.
To confirm the existence of the DBS, we have performed coupled cluster calculations40 for electron attachment. The dipole moment of FNH3 was calculated using the density functional theory (DFT) with dispersion correction (see Supplementary Computational Methods for details). Our calculations showed the existence of a σ-type DBS with a binding energy of 327 and 415 cm−1 for FNH3 in its ground and vHF = 1 states, respectively, with the corresponding dipole moments of 4.8 and 5.0 D. The DBS electron locates mostly at the positively charged end −NH2, as shown by the purple lobe of the corresponding molecular orbital in Fig. 1.
In Fig. 3, the experimental spectra are compared with the theoretical ones. The experimental spectrum in the low-energy wing, which is spliced from high-resolution parts of the multiple spectra in Fig. 2, represents the key finding of the current work. At higher energies, the current spectrum is in good agreement with that reported earlier by Babin et al.25 dominated by direct photodetachment. The theoretical spectra are shifted 183 cm−1 higher in binding energy to match the experiment. By comparing the experimental and theoretical spectra, the peaks can be assigned. In particular, the (0a–0j) and (1a–1f) bands correspond to bound and Feshbach resonance states associated with vHF = 0 and 1, respectively. However, the experimental intensity of the vHF = 0 band is roughly the same as that for vHF = 1, significantly different from the theoretical prediction based on direct photodetachment. This is strong and additional evidence for the DBS enhancement of the vHF = 0 detachment.
The top spectrum in gray represents the overview of experimental cryo-SEVI results. Below it, the high-resolution experimental spectra are shown in black. The raw theoretical spectrum (dark red) as well as the convoluted one (red, a 50-cm−1 FWHM Gaussian function) are plotted for comparison at the bottom. The theoretical spectra are shifted 183 cm−1 higher in the binding energy to match the experiment. The vertical dotted lines (blue) indicate the assignment of vibrational states. v6 is the NH2 out-of-plane wag mode, v8 is the H2N-HF stretching mode. The bold black arrow represents the product asymptote. The theoretical intensity of the band vHF = 0 was multiplied by a factor of 10 for a better comparison. Numbers 0–2 in the labels represent the peaks assigned to the band vHF = 0–2.
The band head peak labeled as 0a corresponds to the transition from the ground state of the FNH3− anion to the ground state of the neutral FNH3 complex. The experimental identification of the ground vibrational state of FNH3 allowed an accurate determination of its electron affinity (EA) for the first time. Its experimental EA value was determined as 20,220(89) cm−1, which agrees well with the theoretical prediction of 20,089 cm−1. The fundamental frequency of the vHF mode in the FNH3 complex was experimentally determined to be 3493 cm−1 by comparing the positions of peaks 0a and 1a. This is also in excellent agreement with the theoretical result of 3470 cm−1. Supplementary Table 1 compares experimental and theoretical peak shifts for FNH3− detachment. The theoretical prediction of the shifts of the resonances agrees well with the experimental spectra. On the other hand, the predicted intensities of the vHF = 0 resonances are in poor agreement with the experiment, even though the agreement for resonances in the vHF ≥ 1 bands is quite good, again underscoring the DBS-mediated mechanism in the former.
Discussion
The large dipole moment of the FNH3 complex (4.8 D) is sufficient to support a DBS. By tuning the photon energy, the FNH3− anion in the ground state can be resonantly excited to the DBS. Furthermore, the binding energy of the DBS electron for vHF = 1 (415 cm−1) is significantly smaller than the vibrational energy. The vibrationally excited neutral core can autodetach due to vibronic coupling. This results in the ejection of the DBS electron, while the neutral core releases one vibrational quantum and returns to vHF = 0, as depicted in Fig. 1. This photoexcitation process is often accompanied by the excitation of vibrational HF stretching mode (vHF), NH2 out-of-plane wag mode (v6), and H2N-HF stretching mode (v8) due to the geometry changing. Although the observed peak positions are well reproduced by theory, the anomalous intensities observed in the vHF = 0 band, attributable to the resonant enhancement via the DBS, were not predicted by theory, as the vibronic autodetachment is not included in the theoretical treatment. An explicit simulation of autodetachment demands a quantum mechanical treatment of electron scattering beyond the scope of the current work.
Indeed, the spectra-profile dependence on the photon energy shown in Fig. 2 is likely due to the vibrational-state-specific photoexcitation and the mode competition during autodetachment. A variety of vibrational energy levels of the neutral core of the DBS can be reached when photoexciting the FNH3− anion from its ground state by tuning the photon energy. When several modes were excited simultaneously, the mode that most significantly alters the dipole moment is more likely to be observed. Since the theoretical spectra in Fig. 3 simulate direct photodetachment, their intensities for peaks 0a–0j are understandably quite different from the experimental ones. To explore all possible photoexcitation resonance via the DBS, we conducted a scan of photon energy and recorded the electron signal and the anion beam signal. Interestingly, we did not observe any sharp resonances (refer to Supplementary Fig. 3). This absence of sharp resonances can presumably be attributed to a fast autodetachment process and dense vibrational peaks. In principle, some sharp DBS resonances may show up near the photodetachment threshold of vHF = 0. However, we did not observe notable photoexcitation to the DBS with vHF = 0, possibly due to the larger geometry difference from the FNH3− anion. In the present work, the observed DBS resonances are well above the photodetachment threshold.
To gain a better understanding of the observed spectral features, vibrational wavefunctions of all the observed peaks were calculated. Since peaks 2a–2j have been investigated by Babin et al. in detail25, we will focus here on the low-lying resonances (and bound states). In Fig. 4, we present the wavefunctions for peaks 0a–0j and 1a–1f, superimposed on the neutral PES. In panel 0a, we also include the contour of the anion wavefunction to illustrate their overlap during the photodetachment. It can be seen that the overlaps between the anionic and neutral wavefunctions are generally very poor, resulting in difficulties in detecting them in the direct photodetachment experiment25. From the localized character of these wavefunctions, it is clear that they are metastable (presumably long-lived) resonances and bound states supported by the product well of the F + NH3 reaction. Based on the product asymptote, states with energies below 22,600 cm−1 are bound.
The labels correspond to the observed peaks in Figs. 2 and 3. Wavefunctions are superimposed on the neutral PES contour plotted with respect to the center-of-mass distance between NH2 and HF (R) and H-F bond length (r1), as defined in Supplementary Fig. 1. The purple contours in panel 0a represent the projection of anionic ground-state wave function onto the neutral PES. Peaks 0a–0j are related to the vibrational states with vHF = 0, while peaks 1a–1f for vHF = 1.
The assignment of these Feshbach resonances and bound states is confirmed by analyzing the nodal structure of the wavefunctions. Peaks 0a–0j exhibit no nodes along the HF stretching coordinate (vertical axis in Fig. 4), while peaks 1a–1f have one node, which is consistent with the assignment of vHF = 0 and vHF = 1. The node number along the horizontal axis in Fig. 4 indicates the vibrational quanta of the H2N–HF stretching mode (v8). For example, peaks 0a and 1a are assigned to v8 = 0, while peaks 0c and 1e are v8 = 2. The wavefunctions plotted along other coordinates are displayed in the Supplementary Fig. 2. All observed resonances can be assigned using the HF stretching mode (vHF), NH2 out-of-plane wagging mode (v6), and H2N–HF stretching mode (v8). A detailed assignment is listed in Supplementary Table 1. The excitation of the v6 mode is a result of the NH3 pyramidal-to-planar transition, while vHF and v8 modes arise from changes in the H–F and H2N–HF equilibrium distances, respectively.
In summary, we have investigated the photodetachment of FNH3− using cryo-SEVI spectroscopy and reduced-dimensional quantum dynamical computations on high-level ab initio PESs. We observed a new series of bound states and resonances for the prototypical F + NH3 → HF + NH2 reaction that was not previously reported, especially the series with the ground HF stretching (vHF = 0) vibrational states, which are hard to detect in direct photodetachment. These neutral vibrational resonances/bound states with poor overlaps with the ground anionic state are detected through vibrational-state-specific photoexcitation of a DBS of FNH3− followed by autodetachment. Our high-level quantum dynamical calculations reproduced the energies of these bound and resonance states, allowing unambiguous assignment. Our results demonstrated a novel approach for probing the dynamics and spectroscopy of the transition-state region of a neutral reaction via a DBS, which is commonly present for polar species. It is worth noting that negative ions rarely have bound excited states; therefore, the existence of a DBS provides an opportunity for the time-resolved observations of reaction dynamics through pump-probe experiments. These transition-state spectroscopy studies are expected to provide a unique and complementary perspective of reaction dynamics to the molecular-beam approach.
Methods
Experimental Methods
The experiment was carried out on the Cryo-SEVI spectrometer41,42. In this work, a strong FNH3− anion beam was generated by expanding a mixed gas of NF3 and NH3 (~1:2) through a pulsed valve fitted with a high-voltage discharge ion source. The backing pressure was around 6 × 105 Pa. The anions were guided by a radiofrequency (rf) hexapole guide and accumulated in a rf octupole ion trap held at 15 K41 and cooled by collisions with the buffer gas (20% H2 and 80% He) for 45 ms. After thermalization to their ground vibrational and electronic state, with only a few lower-lying rotational excited states, the anions were extracted from the trap and mass-selected by time-of-flight43. The anions were then photodetached at various photon energies with tunable light from an optical-parametric-oscillator (OPO) laser pumped by the third harmonic of a neodymium:yttrium–aluminum–garnet (Nd:YAG) laser. The outgoing photoelectrons were projected onto a microchannel-plate enhanced phosphor screen by the static electric field applied by a set of velocity-map-imaging (VMI) lens44,45. The electron-hitting positions were recorded via a charge-coupled device (CCD) camera in an event-count mode and accumulated for typically 50,000 laser shots while running the spectrometer at a 20-Hz repetition rate. The photoelectron distribution was reconstructed from the projected imaging via the maximum entropy velocity Legendre reconstruction (MEVELER) method46. The VMI spectrometer features a high resolution for slow electrons, typically a few cm−1 near the photodetachment threshold. A series of high-resolution spectra at different photon energies were concatenated to produce a full spectrum.
To search for the resonant photoexcitation, our spectrometer can be switched from the standard VMI mode to the scanning mode47. In the scanning mode, the phosphor screen was used as a charged particle detector. A high-speed oscilloscope was connected to the phosphor screen to record both the photoelectron signals and the residual anion signals after photodetachment on account of the different arriving times. To compensate for intensity fluctuations of the anion beam, the ratio of the intensity of the photoelectron signal to the intensity of the residual anion beam was monitored as a function of the scanned wavelength.
Computational methods
The reactive system studied in this work is characterized by diatom–triatom (AB-CDE) Jacobi coordinates, as shown in Supplementary Fig. 1. Since the two NH bonds in the NH2 moiety (NEHCHD) keep mostly unchanged from the anion minimum to the neutral H2N·HF well (from 1.019 to 1.021 Å at the UCCSD(T)-F12/aug-cc-pVTZ level)48, they are expected to be spectators and should be nonactive in the process of photodetachment. Therefore, the lengths of the two non-reactive NH bonds are fixed at 1.93 \({a}_{0}\) in the dynamics calculations, i.e., r2 and r3 in the Jacobi coordinates are fixed at \({r}_{2e}=1.97{a}_{0}\) and \({r}_{3e}=1.93{a}_{0}\), respectively. Here \({a}_{0}\) is the Bohr radius. Within this constraint, the full-dimensional Hamiltonian is reduced to a seven-dimensional one that can be written as (\(\hslash=1\) hereafter)49:
where R, r1, r2, and r3 are defined as the distance between centers of mass of HAFB and HCHDNE, the interatomic distance of HAFB, the distance from HC to the center of mass of HDNE, and the interatomic distance of HDNE, respectively, and the corresponding reduced masses are denoted by \({\mu }_{{\rm {R}}}\), \({\mu }_{1}\), \({\mu }_{2}\), and \({\mu }_{3}\). \({\hat{j}}_{1}\) is the rotational angular momentum operator of HAFB, \({\hat{l}}_{2}\) is the orbital angular momentum operator along r2, and \({\hat{j}}_{3}\) is the rotational angular momentum operator of HDNE. \(\hat{J}\) is coupled by \({\hat{j}}_{1}\), \({\hat{l}}_{2}\) and \({\hat{j}}_{3}\).
The photodetachment of an anion can be reasonably approximated by the Condon model, which assumes that the electron detachment is much faster than nuclear motion. Within this approximation, the photoelectron spectrum is characterized by the squared overlap between the anionic wavefunction and the neutral scattering (or bound state) wavefunction. In this work, the initial wave packet is the ground vibrational state of the anion obtained by diagonalizing the seven-dimensional Hamiltonian on the anion PES. The wave packet is then propagated on the neutral PES using the Chebyshev propagator50. The energy spectrum is computed by cosine Fourier transforming the Chebyshev autocorrelation function. Further details of the quantum dynamics calculations can be found in Supplementary Computational Methods.
More than 41,000 energy points sampled in the relevant configuration space of the F + NH3 reaction were calculated at the level of the unrestricted coupled cluster with singles, doubles, and perturbative triples with Dunning’s augmented correlation-consistent polarized valence triple zeta basis set and core electrons frozen (UCCSD(T)-F12/aug-cc-pVTZ)48, and were then fitted by the fundamental-invariant neural network (FI-NN) method51 to generate the globally accurate neutral PES. The anion PES was developed by fitting a total of ~11,000 energy points at the same level of theory as the neutral PES25.
The dipole moment of FNH3 was calculated using the dispersion-corrected double hybrid density functional theory at the level of B2PLYP-D3/aug-cc-pVTZ. The dipole moment of FNH3 at its ground state was calculated using the equilibrium geometry, while the vibrationally averaged geometry was used to calculate the dipole moment for vHF = 1. The DBS of FNH3 was calculated using the equation of motion for electron attachment coupled cluster method with single and double excitations (EOM-EA-CCSD)40. To accurately describe the diffusive nature of DBS, extra diffusive functions 6s6p in an even-tempered manner were added to the standard aug-cc-pVTZ basis sets for each atom. Further details of DBS calculations can be found in Supplementary Computational Methods.
Data availability
The raw spectral and calculated data of figures generated in this study have been provided in the figshare database and can be obtained at https://doi.org/10.6084/m9.figshare.2543389652. The measured data of all peaks and the numerical parameters used in the calculation in this study are provided in the Supplementary Information.
Code availability
The associated codes, such as the subroutine to generate anion and neutral PESs and the quantum scattering code, are available on GitHub at https://github.com/apmtcc/AB-CDE53 and described in the README file.
References
Eyring, H. The activated complex in chemical reactions. J. Chem. Phys. 3, 107–115 (1935).
Polanyi, J. C. & Zewail, A. H. Direct observation of the transition state. Acc. Chem. Res. 28, 119–132 (1995).
Truhlar, D. G., Garrett, B. C. & Klippenstein, S. J. Current status of transition-state theory. J. Phys. Chem. 100, 12771–12800 (1996).
Guo, H. & Liu, K. Control of chemical reactivity by transition-state and beyond. Chem. Sci. 7, 3992–4003 (2016).
Herschbach, D. R. Reactive collisions in crossed molecular beams. Discuss. Faraday Soc. 33, 149–161 (1962).
Lee, Y. T., McDonald, J. D., LeBreton, P. R. & Herschbach, D. R. Molecular beam reactive scattering apparatus with electron bombardment detector. Rev. Sci. Instrum. 40, 1402–1408 (1969).
Zare, R. N. Laser control of chemical reactions. Science 279, 1875–1879 (1998).
Liu, K. P. Vibrational control of bimolecular reactions with methane by mode, bond, and stereo selectivity. Annu. Rev. Phys. Chem. 67, 91–111 (2016).
Yang, X. State-to-state dynamics of elementary bimolecular reactions. Annu. Rev. Phys. Chem. 58, 433–459 (2007).
Ervin, K. M., Ho, J. & Lineberger, W. C. A study of the singlet and triplet states of vinylidene by photoelectron spectroscopy of H2C=C−, D2C=C−, and HDC=C−. Vinylidene–acetylene isomerization. J. Chem. Phys. 91, 5974–5992 (1989).
Wenthold, P. G., Hrovat, D. A., Borden, W. T. & Lineberger, W. C. Transition-state spectroscopy of cyclooctatetraene. Science 272, 1456–1459 (1996).
DeVine, J. A. et al. Encoding of vinylidene isomerization in its anion photoelectron spectrum. Science 358, 336–339 (2017).
Manolopoulos, D. E. et al. The transition state of the F + H2 reaction. Science 262, 1852–1855 (1993).
Neumark, D. M. Transition state spectroscopy. Science 272, 1446–1447 (1996).
Weichman, M. L. & Neumark, D. M. Slow photoelectron velocity-map imaging of cryogenically cooled anions. Annu. Rev. Phys. Chem. 69, 101–124 (2018).
Metz, R. B., Weaver, A., Bradforth, S. E., Kitsopoulos, T. N. & Neumark, D. M. Probing the transition state with negative ion photodetachment: the Cl + HCl and Br + HBr reactions. J. Phys. Chem. 94, 1377–1388 (1990).
Neumark, D. M. Transition-state spectroscopy via negative ion photodetachment. Acc. Chem. Res. 26, 33–40 (1993).
Neumark, D. M. Probing the transition state with negative ion photodetachment: experiment and theory. Phys. Chem. Chem. Phys. 7, 433–442 (2005).
Continetti, R. E. & Guo, H. Dynamics of transient species via anion photodetachment. Chem. Soc. Rev. 46, 7650–7667 (2017).
Chen, B. et al. The negative ion photoelectron spectrum of cyclopropane-1,2,3-trione radical anion, (CO.)3•−—a joint experimental and computational study. J. Am. Chem. Soc. 136, 12345–12354 (2014).
Otto, R. et al. Imaging dynamics on the F + H2O → HF + OH potential energy surfaces from wells to barriers. Science 343, 396–399 (2014).
Wang, T. et al. Dynamical resonances in chemical reactions. Chem. Soc. Rev. 47, 6744–6763 (2018).
Kim, J. B. et al. Spectroscopic observation of resonances in the F + H2 reaction. Science 349, 510–513 (2015).
Weichman, M. L. et al. Feshbach resonances in the exit channel of the F + CH3OH → HF + CH3O reaction observed using transition-state spectroscopy. Nat. Chem. 9, 950–955 (2017).
Babin, M. C. et al. Observation of resonances in the transition state region of the F + NH3 reaction using anion photoelectron spectroscopy. Nat. Chem. 15, 194–199 (2023).
Hock, C., Kim, J. B., Weichman, M. L., Yacovitch, T. I. & Neumark, D. M. Slow photoelectron velocity-map imaging spectroscopy of cold negative ions. J. Chem. Phys. 137, 224201 (2012).
Neumark, D. M. Slow electron velocity-map imaging of negative Ions: applications to spectroscopy and dynamics. J. Phys. Chem. A 112, 13287–13301 (2008).
Desfrançois, C., Abdoul-Carime, H. & Schermann, J. P. Ground-state dipole-bound anions. Int. J. Mod. Phys. B 10, 1339–1395 (1996).
Qian, C. H., Zhu, G. Z. & Wang, L. S. Probing the critical dipole moment to support excited dipole-bound states in valence-bound anions. J. Phys. Chem. Lett. 10, 6472–6477 (2019).
Liu, H. T., Ning, C. G., Huang, D. L., Dau, P. D. & Wang, L. S. Observation of mode-specific vibrational autodetachment from dipole-bound states of cold anions. Angew. Chem. Int. Ed. 52, 8976–8979 (2013).
Lu, Y., Tang, R. & Ning, C. Observation of an excited dipole-bound state in a diatomic anion. J. Phys. Chem. Lett. 12, 5897–5902 (2021).
Lu, Y., Tang, R., Zhang, R. & Ning, C. Probing isomerization dynamics via a dipole-bound state. J. Phys. Chem. Lett. 13, 8711–8716 (2022).
Kang, D. H., An, S. & Kim, S. K. Real-time autodetachment dynamics of vibrational Feshbach resonances in a dipole-bound state. Phys. Rev. Lett. 125, 093001 (2020).
Kang, D. H., Kim, J., Noh, H. R. & Kim, S. K. Observation of the ponderomotive effect in non-valence bound states of polyatomic molecular anions. Nat. Commun. 12, 7098 (2021).
Rogers, J. P., Anstöter, C. S. & Verlet, J. R. R. Ultrafast dynamics of low-energy electron attachment via a non-valence correlation-bound state. Nat. Chem. 10, 341–346 (2018).
Bull, J. N. & Verlet, J. R. R. Observation and ultrafast dynamics of a nonvalence correlation-bound state of an anion. Sci. Adv. 3, e1603106 (2017).
Clarke, C. J. & Verlet, J. R. R. Dynamics of anions: from bound to unbound states and everything In between. Annu. Rev. Phys. Chem. 75, 89–110 (2024).
Song, H. & Guo, H. Theoretical insights into the dynamics of gas-phase bimolecular reactions with submerged barriers. ACS Phys. Chem. Au 3, 406–418 (2023).
Wigner, E. P. On the behavior of cross sections near thresholds. Phys. Rev. 73, 1002–1009 (1948).
Sneskov, K. & Christiansen, O. Excited state coupled cluster methods. WIREs Comput. Mol. Sci. 2, 566–584 (2012).
Tang, R., Fu, X. & Ning, C. Accurate electron affinity of Ti and fine structures of its anions. J. Chem. Phys. 149, 134304 (2018).
Zhang, R., Lu, Y., Tang, R. & Ning, C. Electron affinity of atomic scandium and yttrium and excited states of their negative ions. J. Chem. Phys. 158, 084303 (2023).
Wiley, W. C. & McLaren, I. H. Time‐of‐flight mass spectrometer with improved resolution. Rev. Sci. Instrum. 26, 1150–1157 (1955).
León, I., Yang, Z., Liu, H. T. & Wang, L. S. The design and construction of a high-resolution velocity-map imaging apparatus for photoelectron spectroscopy studies of size-selected clusters. Rev. Sci. Instrum. 85, 083106 (2014).
Eppink, A. T. J. B. & Parker, D. H. Velocity map imaging of ions and electrons using electrostatic lenses: application in photoelectron and photofragment ion imaging of molecular oxygen. Rev. Sci. Instrum. 68, 3477–3484 (1997).
Dick, B. Inverting ion images without abel inversion: maximum entropy reconstruction of velocity maps. Phys. Chem. Chem. Phys. 16, 570–580 (2014).
Tang, R. et al. Observation of electric-dipole transitions in the laser-cooling candidate Th− and its application for cooling antiprotons. Phys. Rev. A 103, 042817 (2021).
Tian, L., Zhu, Y., Song, H. & Yang, M. Theoretical study of the F(2P) + NH3 → HF + NH2 reaction on an accurate potential energy surface: dynamics and kinetics. Phys. Chem. Chem. Phys. 21, 11385–11394 (2019).
Song, H., Zhu, Y., Pan, M. & Yang, M. Dissociative photodetachment of H3O2−: a full-dimensional quantum dynamics study. Phys. Chem. Chem. Phys. 23, 22298–22304 (2021).
Chen, R. & Guo, H. Evolution of quantum system in order domain of Chebyshev operator. J. Chem. Phys. 105, 3569–3578 (1996).
Shao, K., Chen, J., Zhao, Z. & Zhang, D. H. Communication: fitting potential energy surfaces with fundamental invariant neural network. J. Chem. Phys. 145, 071101 (2016).
Zhang, R., Yan, S., Song, H., Guo, H. & Ning, C. Probing resonances in the transition-state region of the F + NH3 reaction via a dipole-bound state https://doi.org/10.6084/m9.figshare.25433896 (2024).
Song, H. Quantum scattering code to simulate photodetachment process https://doi.org/10.5281/zenodo.10951846 (2024).
Acknowledgements
We thank the National Natural Science Foundation of China (NSFC) (Grant Nos. 12374244 and 11974199 to C.N., 21973109 and 21921004 to H.S.). S.Y. thanks the China Postdoctoral Science Foundation (Grant no. GZC20231367). H.G. is supported by the US Air Force Office of Scientific Research (Grant no. FA9550-22-1-0350).
Author information
Authors and Affiliations
Contributions
The experiments were conceived by C.N. and carried out by R.Z. and S.Y. The data analysis was performed by R.Z. with support from C.N. The calculations were conceived by H.S. and H.G. and performed and analyzed by H.S. with assistance from H.G. The paper was written by R.Z., H.S., H.G. and C.N.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Gabor Czako, who co-reviewed with Dora Papp, and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zhang, R., Yan, S., Song, H. et al. Probing the activated complex of the F + NH3 reaction via a dipole-bound state. Nat Commun 15, 3858 (2024). https://doi.org/10.1038/s41467-024-48202-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-024-48202-7
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.