News & Views |
Featured
-
-
Article |
 Isolation of a californium(II) crown–ether complex
Isolation of a californium(II) crown–ether complex
Californium is difficult to prepare in its divalent state. Now, crystals of a Cf(II) crown–ether complex have been synthesized by reduction of a Cf(III) precursor with an Al/Hg amalgam. They exhibit 5f→6d transitions in the visible region and near-infrared emission that are highly sensitive to changes in the coordination environment.
- Todd N. Poe
- , Harry Ramanantoanina
- & Cristian Celis-Barros
-
Article |
 Polyoxometalates as ligands to synthesize, isolate and characterize compounds of rare isotopes on the microgram scale
Polyoxometalates as ligands to synthesize, isolate and characterize compounds of rare isotopes on the microgram scale
The study of rare isotopes is hampered by their scarcity, cost and sometimes toxicity. Now polyoxometalate ligands have been shown to facilitate the capture of f-block elements and their characterization. Single-crystal X-ray diffraction structures have been obtained for several molecular complexes, including three of the rare curium-248, from minute amounts (micrograms) of material.
- Ian Colliard
- , Jonathan R. I. Lee
- & Gauthier J.-P. Deblonde
-
Article |
 A 9.2-GHz clock transition in a Lu(II) molecular spin qubit arising from a 3,467-MHz hyperfine interaction
A 9.2-GHz clock transition in a Lu(II) molecular spin qubit arising from a 3,467-MHz hyperfine interaction
The s-orbital mixing into the spin-bearing d orbital associated with a molecular Lu(II) complex is shown to both reduce spin–orbit coupling and increase electron–nuclear hyperfine interactions, which substantially improves electron spin coherence. Combined with the potential to tune interactions through coordination chemistry, it makes this system attractive for quantum information applications.
- Krishnendu Kundu
- , Jessica R. K. White
- & Stephen Hill
-
News & Views |
 Probing precipitation properties
Probing precipitation properties
Superheavy elements are short-lived and only available on a single-atom level, making their chemical properties very challenging to study. Now, through their co-precipitation with samarium, single atoms of rutherfordium have been shown to form hydroxide complexes but not ammine ones.
- Alexander Yakushev
-
Article |
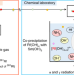 Co-precipitation behaviour of single atoms of rutherfordium in basic solutions
Co-precipitation behaviour of single atoms of rutherfordium in basic solutions
It is difficult to investigate the chemical properties of superheavy elements, which are only available an atom at a time and rapidly decay. A co-precipitation method with samarium has now been developed that suggests rutherfordium would form hydroxide precipitates—but not ammine ones—if it were possible to perform these experiments on macroscopic quantities.
- Yoshitaka Kasamatsu
- , Keigo Toyomura
- & Atsushi Shinohara
-
Article |
 Developing the 134Ce and 134La pair as companion positron emission tomography diagnostic isotopes for 225Ac and 227Th radiotherapeutics
Developing the 134Ce and 134La pair as companion positron emission tomography diagnostic isotopes for 225Ac and 227Th radiotherapeutics
134Ce and 134La have great potential as companion diagnostic isotopes for radiotherapeutics labelled with α-emitting 225Ac and 227Th. Now, by controlling the CeIII/CeIV redox couple, the large-scale production, purification and characterization of 134Ce- and 134La-based radiolabels has been achieved and their use for in vivo positron emission tomography is demonstrated.
- Tyler A. Bailey
- , Veronika Mocko
- & Rebecca J. Abergel
-
Editorial |
 Chronicles of a chemical chart
Chronicles of a chemical chart
As the International Year of the Periodic Table draws to an end, we reflect on how it has prompted chemists to explore the past, present and future of this chemical icon.
-
-
Q&A |
 Weighing up superheavy elements
Weighing up superheavy elements
Jadambaa Khuyagbaatar from the Helmholtz Institute Mainz and the GSI Helmholtz Centre for Heavy Ion Research talks to Nature Chemistry about superheavy element studies and why creating and exploring these fleeting nuclei matters.
- Anne Pichon
-
Editorial |
 End of an elemental era
End of an elemental era
We reflect on our monthly ‘In Your Element’ feature that comes to an end in this issue.
-
In Your Element |
 Mendelevium 101
Mendelevium 101
The first element to be identified one atom at a time was named after the main architect of the modern periodic table. This seemingly straightforward etymological choice illustrates how scientific recognition can eclipse geopolitical tensions, says Anne Pichon.
- Anne Pichon
-
Thesis |
 Isotopic enrichment
Isotopic enrichment
Michelle Francl suggests that we should expand our view of the periodic table to new dimensions.
- Michelle Francl
-
Comment |
 A new period in superheavy-element hunting
A new period in superheavy-element hunting
The periodic table as we know it now seems complete, its current 118 elements nicely fitting in the seven familiar rows. How many more can be synthesized — and how will the table expand to accommodate them? The search for ever-heavier elements is pointing towards new periods, though perhaps not as neatly ordered as the first seven.
- Hiromitsu Haba
-
In Your Element |
 The making of moscovium
The making of moscovium
Yuri Oganessian relates the story of the formation and decay of a doubly odd moscovium nucleus.
- Yuri Oganessian
-
Comment |
 Neutron stardust and the elements of Earth
Neutron stardust and the elements of Earth
At its inception, the periodic table sorted elements by weight, so it may be surprising that the heaviest natural element on Earth remains controversial, or at best, nebulous. In the strange, perhaps-unfinished search for this weightiest nucleus, the only definitive conclusion is that it lies somewhere beyond uranium.
- Brett F. Thornton
- & Shawn C. Burdette
-
In Your Element |
 Promethium puzzles
Promethium puzzles
Stuart Cantrill explains why looking to the heavens for element 61 — named after the Titan who stole fire from the gods — could extend the periodic table.
- Stuart Cantrill
-
In Your Element |
 Targeting tennessine
Targeting tennessine
Liz Williams explores the synthesis of tennessine, a story in which elements in supporting roles play a crucial part.
- Elizabeth Williams
-
In Your Element |
 Roentgenium generation
Roentgenium generation
Taye Demissie relates unununium’s unusually smooth route to roentgenium, and how predicting its properties relies on relativistic calculations.
- Taye B. Demissie
-
In Your Element |
 The realities of radium
The realities of radium
Vikki Cantrill tells the story of element 88’s discovery and how its glowing reputation eventually faded.
- Vikki Cantrill
-
In Your Element |
 The oganesson odyssey
The oganesson odyssey
Kit Chapman explores the voyage to the discovery of element 118, the pioneer chemist it is named after, and false claims made along the way.
- Kit Chapman
-
In Your Element |
 Tritium trinkets
Tritium trinkets
Scientists take nomenclature seriously, but tritium was named in a casual aside. Brett F. Thornton and Shawn C. Burdette discuss the heavy, radioactive hydrogen isotope that is available for purchase online.
- Brett F. Thornton
- & Shawn C. Burdette
-
In Your Element |
 Hidden hassium
Hidden hassium
From its scarcity to political intrigue over naming conventions, element 108’s story describes how international cooperation overcame the limits of nuclear science, says Michael Tarselli.
- Michael A. Tarselli
-
Article |
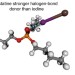 Experimental and computational evidence of halogen bonds involving astatine
Experimental and computational evidence of halogen bonds involving astatine
Halogen bonding is known to get stronger with increasing halogen polarizability, but some trends of the periodic table break down for heavy elements owing to relativistic effects. Now, through distribution coefficient measurements and relativistic quantum mechanical calculations, AtI has been shown to form stronger halogen bonds than I2—meaning that astatine conforms to the trend.
- Ning Guo
- , Rémi Maurice
- & Nicolas Galland
-
In Your Element |
 Nihonium the obscure
Nihonium the obscure
Iulia Georgescu explains her fascination with the elusive element 113.
- Iulia Georgescu
-
In Your Element |
 The darmstadtium cornerstone
The darmstadtium cornerstone
Dieter Ackermann explains why element 110 occupies a significant place in the superheavy corner of the periodic table.
- Dieter Ackermann
-
In Your Element |
 Peaceful berkelium
Peaceful berkelium
The first new element produced after the Second World War has led a rather peaceful life since entering the period table — until it became the target of those producing superheavy elements, as Andreas Trabesinger describes.
- Andreas Trabesinger
-
In Your Element |
 Meitnerium in tribute
Meitnerium in tribute
Adrian Dingle tells the story of how the name of element 109 represents the lasting recognition that one of the greatest nuclear physicists was in danger of never receiving.
- Adrian Dingle
-
In Your Element |
 Frantically forging fermium
Frantically forging fermium
Brett F. Thornton and Shawn C. Burdette relate how element 100 was first identified in a nuclear weapons test, but that was classified information, so researchers had to 'discover' it again using other methods.
- Brett F. Thornton
- & Shawn C. Burdette
-
Article |
 Chelation and stabilization of berkelium in oxidation state +IV
Chelation and stabilization of berkelium in oxidation state +IV
Berkelium is the only transplutonium element predicted to be able to exhibit both +III and +IV oxidation states in solution. Bk(IV) has now been stabilized through chelation with a siderophore derivative. The resulting neutral coordination compound was characterized and compared with the negatively charged species obtained by chelation of neighbouring trivalent actinides.
- Gauthier J.-P. Deblonde
- , Manuel Sturzbecher-Hoehne
- & Rebecca J. Abergel
-
Article |
 Actinide covalency measured by pulsed electron paramagnetic resonance spectroscopy
Actinide covalency measured by pulsed electron paramagnetic resonance spectroscopy
Covalency in actinide–ligand bonding is poorly understood compared to that in other parts of the periodic table due to the lack of experimental data. Here, pulsed electron paramagnetic resonance methods are used to directly measure the electron spin densities at coordinated ligands in molecular thorium and uranium complexes.
- Alasdair Formanuik
- , Ana-Maria Ariciu
- & David P. Mills
-
In Your Element |
 Curious curium
Curious curium
From secretive beginnings to serving in missions on Mars, Rebecca J. Abergel and Eric Ansoborlo take a look at the glowing mark curium has left on contemporary science and technology.
- Rebecca J. Abergel
- & Eric Ansoborlo
-
In Your Element |
 Lawrencium's place at the table
Lawrencium's place at the table
Yuichiro Nagame ponders on the steps it took to make lawrencium, and its location in the periodic table.
- Yuichiro Nagame
-
In Your Element |
 Tantalizing tantalum
Tantalizing tantalum
Giovanni Baccolo relates tales of tantalum, an element known, and named, for its inertness, yet one that holds some surprises, such as a naturally occurring nuclear isomer.
- Giovanni Baccolo
-
In Your Element |
 Californium gleaming
Californium gleaming
Thomas Albrecht-Schmitt explains the origin of element 98's striking green glow, and why the future for californium chemistry is just as bright.
- Thomas Albrecht-Schmitt
-
In Your Element |
 Nobelium non-believers
Nobelium non-believers
Alfred Nobel's eponymous element, nobelium, was 'first' discovered either in the 1950s or 1960s, in the USSR, Sweden or the USA. Brett F. Thornton and Shawn C. Burdette delve into the ensuing decades of internecine strife over the discovery of element 102.
- Brett F. Thornton
- & Shawn C. Burdette
-
Thesis |
 The straight dope on isotopes
The straight dope on isotopes
A century ago this month, Frederick Soddy described and named isotopes in the pages of Nature. Brett F. Thornton and Shawn C. Burdette discuss how chemists have viewed and used isotopes since then — either as chemically identical or chemically distinct species as the need required and technology allowed.
- Brett F. Thornton
- & Shawn C. Burdette
-
In Your Element |
 Enigmatic astatine
Enigmatic astatine
D. Scott Wilbur points out the difficulty in studying the transient element astatine, and the need to understand its basic chemical nature to help in the development of targeted radiotherapy agents.
- D. Scott Wilbur
-
In Your Element |
 Peculiar protactinium
Peculiar protactinium
Richard Wilson relates how the rare, highly radioactive, highly toxic element protactinium puzzled chemists for a long time, and was discovered and named twice from two different isotopes before finding its place in fundamental research.
- Richard Wilson
-
In Your Element |
 Neglected neptunium
Neglected neptunium
Jim Ibers talks about neptunium, an element that has remained largely unnoticed despite the flurry of activity devoted to its neighbours in the periodic table, uranium and plutonium.
- Jim Ibers
-
News & Views |
 A tale of two nitrides
A tale of two nitrides
The first thing that comes to mind about uranium is certainly not its similarity to iron. Chemists have now shown that the two have a lot in common.
- Paula Diaconescu
-
In Your Element |
 The riches of uranium
The riches of uranium
Uranium is best known, and feared, for its involvement in nuclear energy. Marisa J. Monreal and Paula L. Diaconescu take a look at how its unique combination of properties is now increasingly attracting the attention of chemists.
- Marisa J. Monreal
- & Paula L. Diaconescu
-
In Your Element |
 Welcome copernicium?
Welcome copernicium?
In the search for superheavy elements, element 112 was a stepping stone towards the 'islands of stability'. Sigurd Hofmann now relates the steps that led to its 'creation' and detection.
- Sigurd Hofmann
-
In Your Element |
 The race for rutherfordium
The race for rutherfordium
Mitch André Garcia considers the disputed discovery of element 104 and takes a look at how the chemistry of this synthetic element is developing.
- Mitch André Garcia

 Doing away with radium’s proxies
Doing away with radium’s proxies