Abstract
NKG2D is a major activating receptor of NK cells and plays a critical role in tumor immunosurveillance. NKG2D expression in NK cells is inhibited by the histone deacetylase (HDAC) inhibitor valproic acid (VPA) and enhanced by the narrow-spectrum HDAC inhibitor entinostat. We previously demonstrated that entinostat enhanced NKG2D transcription by increasing acetylation of Histones H3 and H4. However, the mechanism by which VPA reduces NKG2D expression in NK cells is not known. We have also shown that NKG2D transcription is regulated by STAT3 phosphorylation. In this study, we investigated regulation of NKG2D expression in NK cells by VPA and entinostat by assessing protein expression, phosphorylation, and interaction of HDACs and STAT3. We find that VPA selectively inhibits STAT3 tyrosine705 phosphorylation, but entinostat does not. STAT3 complexes with HDAC3, and HDAC3 inhibition represses STAT3 phosphorylation and therefore NKG2D expression. NK cells from STAT3 wild-type mice downregulate NKG2D in response to VPA, but not NK cells from STAT3 knockout mice. These results show that VPA is a potent inhibitor of STAT3 phosphorylation and demonstrate that histone acetylation and STAT3 tyrosine705 phosphorylation cooperate in regulating NKG2D expression in NK cells.
Similar content being viewed by others
Introduction
Natural Killer (NK) cells are large granular lymphocytes that play a critical role in the host defense against viral infection and cancers. NK cell-mediated lysis of target cells is determined by the balance of signaling between activating and inhibitory receptors on NK cells1. NKG2D is the major activating receptor of NK cells and plays a crucial role in tumor immunosurveillance2. In humans, NKG2D recognizes and binds to MIC A/B (MHC class I–related chains A and B) and ULBPs (UL16-binding proteins) on target cells and exerts an activating signal to promote NK cell cytotoxicity. NKG2D expression can be enhanced by the cytokines IL-2, -12, -15, and -213, and is suppressed by the inflammatory factors TGF-β, prostaglandin E2 (PGE2) and L-kynurenine, a tryptophan catabolite generated by indoleamine-2,3-dioxygenase (IDO)4,5,6. Enhancement of NKG2D expression on NK cells or of its ligands on cancer cells is a potential strategy for improving cancer immunotherapy.
Histone deacetylases (HDACs) are enzymes catalyzing the removal of acetyl groups, leading to chromatin condensation and transcriptional repression7. HDACs are involved in cell proliferation, apoptosis, differentiation, migration, and metastases in cancers8. Therefore, HDACs have become promising targets for cancer treatment, and several classes of HDAC inhibitors have been developed and have been tested in clinical trials9. HDAC inhibitors (HDACi) such as valproic acid (VPA) and entinostat have been shown to sensitize NK cell-mediated killing by upregulating expression of NKG2D ligands MICA/B or ULBP1on cancer cells10,11,12,13,14,15, suggesting that HDACi might have promising applications in cancer immunotherapy, especially in combination with adoptive NK cell therapy.
However, the HDACi VPA has been found to impair NK cell cytolytic activity by decreasing expression of NKG2D on NK cells16,17. This is in contrast to our finding that the HDACi entinostat enhances NK cell cytolytic activity by increasing expression of NKG2D on NK cells15. We showed that entinostat enhances the expression of NKG2D in NK cells through inhibiting HDAC1 and HDAC2, thereby increasing acetylation of histones H3 and H4 which result in increased NKG2D transcription. VPA also inhibits HDAC1 and HDAC2, so we questioned why VPA reduces the expression of NKG2D in NK cells rather than enhancing it. Understanding the mechanism of VPA-induced suppression of NKG2D expression in NK cells would be valuable for developing HDAC as targeted cancer drugs for NK cell immune modulation.
To elucidate the mechanism by which VPA down-regulates NKG2D expression in NK cells, we assessed the expression and regulation of NKG2D in NK cells after VPA treatment by analyzing protein expression, phophorylation and interaction, using entinostat as a control. We found that HDAC3 was able to be complexed with STAT3; VPA inhibited HDAC3 and then resulted in selectively inhibition of STAT3 tyrosine705 phosphorylation, and subsequent down-regulation of NKG2D expression in NK cells. This finding indicates that HDAC3 and STAT3 signaling cooperate in the regulation of NKG2D expression in NK cells, and provides valuable insight for new drug development and clinical applications of HDACi in cancer treatment.
Results
HDACi VPA reduces but entinostat enhances NKG2D expression in NK cells
To further define the regulation of NKG2D in NK cells by HDACi, we treated primary human NK cells with the wide-spectrum HDACi VPA and the narrow-spectrum HDACi entinostat. We found that VPA ≥ 1 mM and entinostat ≥1 μM significantly impaired NK cell viability, but VPA ≤ 0.5 mM and entinostat ≤0.1 μM did not (Suppl. Fig. 1A,B). Since these values also fall within the published in vitro effective-dose concentrations for these two inhibitors18,19, we treated NK cells with 0.5 mM VPA or 0.1 μM entinostat and found that VPA reduced, but entinostat enhanced NKG2D expression (Fig. 1A). We then analyzed the increasing percentage ratio of NKG2D expression with VPA or entinostat compared to untreated control, we found VPA significantly decreased but entinostat significantly increased NKG2D expression (Fig. 1B). To further determine the effect of VPA, we used varying concentrations of VPA to treat NK cells and found that VPA down-regulated NKG2D expression in NK cells in a dose-dependent manner (Fig. 1C). These results showed that the narrow-spectrum HDACi entinostat and the wide-spectrum HDACi VPA exert opposite effects on NKG2D expression in NK cells.
NK cells were treated with 0.1 μM of entinostat or 0.5 mM of VPA for 24 hrs, NKG2D expression was accessed by flow cytometry. (A) Representative of 5 independent experiments. (B) Percentage increasing of NKG2D expression was calculated by the formula: (NKG2D Mean in treated NK cells - NKG2D Mean in untreated NK cells)/NKG2D Mean in untreated NK cells *100. The data were pooled from 5 donors. (C) Effect of VPA concentration on NKG2D expression. *p < 0.05; **p < 0.01; ***p < 0.001.
VPA impairs NK cell degranulation and cytotoxicity
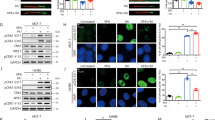
Since VPA down-regulated NKG2D expression in NK cells, we next determined whether VPA impaired NK cell antitumor activity. CD107α expression on the surface of NK cells is a sensitive marker of NK cell functional activity and correlates with NK cell degranulation and cytotoxicity20. We used 0.1 μM entinostat and 0.1 mM of VPA to treat primary human NK cells for 24 h, and then co-incubated NK cells with COL, HCT-15 and SaOS2 cells for 4 h, respectively. We found that entinostat enhanced CD107 surface expression on NK cells, but VPA clearly reduced it (Fig. 2). We also determined the effect of VPA on NK cell cytoxicity by the calcein release assay and found that VPA significantly inhibited NK cell cytotoxicity in a dose-dependent manner (Suppl. Fig. 2). These results showed that VPA has a significant inhibitory effect on NK cell degranualtion and cytotoxicity.
Primary human NK cells were treated with 0.1 μM of entinostat and 0.1 mM of VPA for 24 hrs, respectively; and then co-incubated with COL, HCT-15 and SaOS2 cells (E:T = 4:1) for 4 hrs. NK cell degranulation was evaluated by flow cytometry for CD107a expression. (A) Representative dot plot; (B) Percentage increasing of CD107a expression was calculated by the formula: (percentage CD107a in treated NK cells - percentage CD107a in untreated NK cells)/percentage CD107a in untreated NK cells *100. The data are expressed as mean ± SEM of three independent experiments. *p < 0.05; **p < 0.01; ***p < 0.001.
VPA inhibits STAT3 Tyrosine705 phosphorylation
In our previous study, we found that signal transducer and activator of transcription-3 (STAT3) phosphorylation is critical for the transcriptional expression of NKG2D in NK cells21; this led us to determine the effect of VPA on STAT3 phosphorylation. We treated primary human NK cells for 24 h with 1 μM entinostat or 0.1 mM VPA and then assessed STAT3 phosphorylation. We found that VPA inhibited STAT3 tyrosine705 phosphorylation, and had no effect on total STAT3 protein expression and serine727 phosphorylation. Entinostat had no effect on STAT3 protein expression or phosphorylation (Fig. 3A). To further determine the selective action of VPA on STAT3 inhibition, we measured the phosphorylation of STAT1 and STAT5 in NK cells after treatment with VPA and found that VPA did not affect STAT1 and STAT5 phosphorylation (Fig. 3B). These results indicated that VPA selectively inhibits STAT3 tyrosine705 phosphorylation, and suggested that down-regulation of NKG2D expression by VPA in NK cells might be mediated through inactivation of STAT3.
(A) Expression and phosphorylation of STAT3 in purified primary NK cells with or without treatment with 1.0 μM entinostat or 0.1 mM VPA. (B) Phosphorylation of STAT1 and STAT5 in purified primary NK cells with or without treatment with 0.1 mM VPA and IL-2. Similar results were obtained in three independent experiments.
VPA-induced down-regulation of NKG2D expression is abrogated by STAT3 deletion
As the above results showed that VPA inhibited phophorylation of STAT3 tyrosine705 in NK cells, we next determined whether STAT3 is necessary for VPA-inducted down-regulation of NKG2D. We assessed NKG2D expression in NK cells from mice with conditional deletion of floxed STAT3 limited to hematopoietic cells through Tie2 activation of Cre. We found that NKG2D expression was significantly lower in NK cells from STAT3 KO mice compared to WT mice. VPA could significantly down-regulate NKG2D expression in NK cells from STAT3 WT mice, but did not decrease NKG2D expression in NK cells from STAT3 KO mice (Fig. 4). These results demonstrate that STAT3 is required for VPA-induced down-regulation of NKG2D expression in NK cells.
Murine NK cells were isolated from spleens of STAT3 wild-type and knockout mice, and then treated with/without 0.1 mM VPA for 24 hrs. NKG2D surface expression on CD3-NKp46+ cells was analyzed by flow cytometry. The data was pooled from 4 mice. *p < 0.05; **p < 0.01; ***p < 0.001; NS, non-significant.
STAT3 interacts with HDAC3
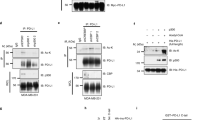
Having demonstrated that VPA is an HDAC inhibitor that also inhibits STAT3 phosphorylation, we questioned whether HDAC deacetylase activity is required for STAT3 phosphorylation. VPA mainly inhibits the class I (HDAC1, 2, 3 and 8) and class IIa (HDAC4, 5, 7 and 9) HDACs9, and inhibits class I more efficiently than class II enzyme HDACs 5 and 619. By a prelimary screening (data not shown), we found that STAT3 could be pull-downed by HDAC3, which suggested HDAC3 might be complexed with STAT3. To further confirm this issue, we first used HDAC3 antibody to pull-down and then immunoblotted with STAT3, we found HDAC3 pull-down actually had a clear STAT3 band (Fig. 5A). We then immunoprecipitated STAT3 followed by immunoblotting with HDAC3 antibody. We found that STAT3 and HDAC3 clearly co-precipitated compared to isotype IgG (Fig. 5B). Since STAT3 could bind with HDAC3, this suggested that HDAC3 might play a role in STAT3 phosphorylation. To assess this functional interaction we treated human NK cells with the HDAC3-specific inhibitor RGFP96622. We found that RGFP966 inhibited the phosphorylation of STAT3 (Fig. 5C), but neither VPA nor RGFP966 affected the acetylation of STAT3. These results show that STAT3 complexes with HDAC3 in NK cells, and HDAC3 is required for STAT3 phosphorylation.
(A) Proteins were immunoprecipitated from NK cell lysate by HDAC3 monoclonal antibody and isotype IgG, and then detected by STAT3 and HDAC3 antibodies using two separate blots with the same sample to avoid the HDAC3 (49 kDa) to be overlapped with the antibody heavy IgG (55 kDa). (B) Proteins were immunoprecipitated from NK cell lysate by STAT3 monoclonal antibody and isotype IgG, and then detected by HDAC3 and STAT3 antibodies using two separate blots with the same sample. (C) NK cells were treated with Entinostat, VPA and RGFP966. Phosphorylation, acetylation and total protein of STAT3 and acetylated histone H3/H4 were detected by western-blot. Similar results were obtained in three independent experiments.
HDAC3 inhibition down-regulates NKG2D expression
Having determined that HDAC3 was required for STAT3 phosphorylation and having previously shown that STAT3 phosphorylation is required for NKG2D expression in NK cells, we next determined the effect of HDAC3 on NKG2D expression. We used the HDAC3-specific inhibitor RGFP96622, HDAC6-specific inhibitor CAY1060323, and HDAC8-specific inhibitor PCI3405124 to treat expanded NK cells. We found that VPA ≤ 0.5 mM, RGFP966 ≤ 20 μM, CAY10603 ≤ 50 nM and PCI34051 ≤ 0.625 μM did not impair NK cell viability at 24 h (Suppl. Fig. 3). The HDAC3 inhibitor RGFP966 caused down-regulation of NKG2D expression on NK cells in a dose-dependent manner similar to VPA, but the HDAC6 inhibitor CAY10603 and the HDAC8 inhibitor PCI34051 did not decrease NKG2D expression (Fig. 6). These results show that HDAC3 inhibition down-regulates NKG2D expression in NK cells and indicates that HDAC3 is required for NKG2D expression.
Discussion
In this study, we sought to identify the mechanism by which VPA decreases expression of NKG2D in NK cells. We found that VPA reduced but entinostat enhanced expression of NKG2D and NK cell degranulation. We also found that VPA inhibited STAT3 tyrosine705 phosphorylation but entinostat did not, suggesting that STAT3 phosphorylation was critical for NKG2D expression in NK cells. We then demonstrated that STAT3 interacts with HDAC3, and that HDAC3 inhibition represses STAT3 tyrosine705 phosphorylation - and therefore NKG2D expression - in NK cells. Furthermore, VPA-induced down-regulation of NKG2D is abrogated by STAT3 deletion. These results clearly show that VPA-mediated inhibition of NKG2D expression in NK cells is dependent on both STAT3 and HDAC3.
STAT3 promotes tumorigenesis as an oncogene by regulating the expression of various target genes that mediate cell cycle, survival, proliferation, apoptosis, invasion, angiogenesis, and metastasis25. STAT3 also promotes tumor immune escape by governing multiple immunosuppressive mechanisms including macrophage polarization to the M2 phenotype, inhibition of DC development, and accumulation of immunosuppressive cells such as Tregs, Th17 cells, and MDSCs26. Therefore, a strong interest has developed in STAT3 as a potent therapeutic target for cancer treatment. A number of STAT3 inhibitors have been developed and tested in clinical trials, but so far no STAT3-specific inhibitor has been approved for clinical use27.
In our previous study, we found that STAT3 is required for NKG2D expression and cytotoxicity in human NK cells21. In the current study, we demonstrated that VPA inhibits phosphorylation of STAT3 and thereby reduces expression of NKG2D in NK cells from wild-type mice, but not in NK cells from STAT3 knockout mice. These results indicate that STAT3 is required for NK cell-mediated immunosurveillance through modulation of NKG2D.
In the current study, we found that STAT3 interacts with HDAC3, and HDAC3 inhibition by VPA or the HDAC3-specific inhibitor RGFP669 reduced expression of NKG2D in NK cells. This shows that HDAC3 regulates NKG2D expression in NK cells, confirming the regulation of STAT3 by HDAC3 that was recently demonstrated in diffuse large B cell lymphoma28. In lymphoma, inhibition of HDAC activity by panobinostat (LBH589) increased STAT3 Lys685 acetylation28, but in our study we found that entinostat, VPA and RGFP669 did not affect STAT3 acetylation. This may be due to the difference of B cells and NK cells, or more broadly of cancer cells and normal cells.
We showed that NKG2D expression in NK cells may be regulated by the phosphorylation of STAT3 in this study, and by the acetylation of Histones-H3 and H4 in our previous study15 (Fig. 7). Entinostat preferentially inhibits HDAC1, is less active against HDAC3, and only marginally inhibits HDAC829, in contrast to VPA inhibits the class I(HDAC1, 2, 3 and 8) and class IIa (HDAC4, 5, 7 and 9) HDACs9. However, VPA decreases but entinostat enhances NKG2D expression, which suggests that regulation of NKG2D expression in NK cells is more dependent on HDAC3-STAT3 signaling than HDAC1-Ac-H3/4 signaling.
HDAC3 is required for STAT3 phospharylation which determines NKG2D transcript in NK cells. VPA inhibits HDAC3 and results in suppression of STAT3 phosphorylation and then reduction of NKG2D expression in NK cells. Histones-H3 and H4 can be acetylated by HAT, and acetylated-H3 and H4 can be deacetylated by HDAC. Entinostat preferentially inhibits HDAC1 and is less active against HDAC3 resulting in enhanced acetylation of H3 and H4 and then increased binding of acetylated H3 and H4 (Ac-H3, Ac-H4) to the promoter of NKG2D, promoting NKG2D transcription.
We assessed the expression and regulation of NKG2D in NK cells by HDAC inhibitors VPA and entinostat, Taken together, we found that VPA inhibits HDAC3 resulting in selective inhibition of phosphorylation of STAT3 tryrosine705, which down-regulates NKG2D expression in NK cells. These findings show that HDAC3-STAT3 signaling regulates NKG2D expression in NK cells, suggesting that the development of new cancer drugs should avoid impairing this signal pathway.
Methods
Reagents
Murine monoclonal antibodies against human CD3, CD56, CD107a, and NKG2D, antibodies against murine CD3, NKp46, and NKG2D, and isotype control monoclonal antibodies were obtained from BioLegend Inc. (San Diego, CA). The recombinant human IL-2 was obtained from PeproTech (Rehovot, Israel). Entinostat, VPA, PCI24781, RGFP966, CAY10603 and PCI34051were obtained from Selleck (Texas, USA). Antibodies against human pSTAT1 (Tyr 701), STAT3, pSTAT3 (Tyr 705), p-STAT3 (Ser727), pSTAT5 (Tyr 694), HDAC1–9 and β-actin were obtained from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). Cell Counting Kit (CCK8) was purchased from YESAN (Shanghai, China). Calcein-AM was purchased from Sigma-Aldrich (St. Louis, MO).
Cell culture
COL (neuroblastoma) and HCT-15 (colon carcinoma) were obtained as previously described and cultured in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum and 1% penicillin and streptomycin. SaOS2 cells from ATCC were cultured in RPMI1640 medium (Gibco) supplemented with 10% of fetal calf serum (Gibco), 1% of penicillin-streptomycin, and 2 mM of L-Glutamine in 5% CO2 at 37 °C.
NK cell purification
Human peripheral blood mononuclear cells (PBMC) were obtained from the Shanghai Blood Center under a research protocol approved by the Department of Shanghai Blood Administration. Human NK cells were purified from PBMC using the RosetteSep Human NK Cell Enrichment Cocktail (StemCell Technologies, Vancouver, BC, Canada) as described previously30. Murine splenocytes were obtained from wild-type (WT) mice or mice in which floxed STAT3 was deleted in hematopoietic cells by Cre expression under the Tie2 promoter31. Murine NK cells were isolated from splenocytes using the EasySep Mouse NK Cell Enrichment Kit (Stemcell Technologies Vancouver, BC, Canada). All experiments involving animals were conducted in accordance with Institutional Animal Care and Use Committee approval at University of Texas MD Anderson Cancer Center.
Human NK cell expansion
Human NK cells were expanded from PBMC in vitro by weekly stimulation with mbIL-21-CD137L-K562 cells in the presence of 100 U/mL of IL-2 for 2–3 weeks as described previously32. PBMCs were either freshly used or frozen in 10% of DMSO containing FBS. For frozen PBMCs, PBMCs were thawed 1 day prior to their cultivation in RPMI 1640 medium supplemented with 10% of FBS, 1% of penicillin-streptomycin, 2 mM of L-Glutamine and 200 U/ml of IL-2 in 5% CO2 at 37 °C.
Cell viability
To determine the effect of VPA, PCI24781, RGFP966, CAY10603 and PCI34051 on the viability of NK cells, CCK8 assay was performed. 1 × 104 of NK cells were seeded per well in 96-well plates. VPA, PCI24781, RGFP966, CAY10603 and PCI34051 were added to the indicated concentration. 24 hrs later, 10 μL of CCK8 was added per well. Plates were incubated for 1 hr and then read at 450 nm using Synergy 2 Multi-Mode Microplate Reader (BioTek Instrument, Int., Winooski, VT). Independent experiments at least were performed three times.
Degranulation Assay
To evaluate the effect of VPA on NK cell degranulation, CD107α surface expression on NK cells was analyzed. NK cells were treated with 0.1 mM of VPA or 0.1 μM of entinostat for 24 hrs, and then cocultured with COL, HCT-15 or SaOS2 cells at a 4:1 ratio with anti–human CD107α or isotype control antibodies present during the coculture period. After 4 hrs co-incubation, cells were stained with anti-human CD56, and NK cell degranulation (CD56+CD107α+) was assessed by flow cytometry.
Flow Cytometry
Cells were exposed to appropriate fluorescence-conjugated antibodies for 30 min at 4 °C in the dark, and then washed and resuspended in 1% FBS containing PBS. Data was acquired by a BD Accuri C6 (BD Biosciences), and analyzed using FlowJo software (Ashland, OR).
Calcein Release Assay
NK cell tumoricidal activity was determined by the calcein release assay as described previously33. Briefly, primary NK cells were incubated with VPA or DMSO for 24 hrs. Target cells were labeled with 2 μg/mL of calcein-AM for 1 hr at 37 °C with occasional shaking. Effector cells and target cells were cocultured at 40:1 ratio and incubated at 37 °C for 4 hrs. After incubation, 100 μL of the supernatant was harvested and transferred to a new plate. Absorbance at 570 nm was determined using a Synergy 2 Multi-Mode Microplate Reader (BioTekInstrument, Int., Winooski, VT). Percent lysis was calculated according to the formula [(experimental release-spontaneous release)/(maximum release − spontaneous release)] × 100.
Western Blot Analysis
NK cells were incubated as indicated with entinostat (1.0 μM), VPA (0.1 mM), RGFP966 (0, 2.5, 5.0, 10 and 20 uM) for 24 hrs and then were lysed with 50 mM Tris-Cl (pH 6.8), 100 mM dithiothreitol, 2% SDS, and 10% glycerol. Samples were quantitated using BCA protein reagent assay kit (Yeasen, Shanghai) and analyzed by 8~10% of SDS-PAGE, followed by immunoblotting using Enhanced Chemiluminescence Substrate (MerckMillipore, USA) according to the manufacturer’s instructions. Bands were visualized using a chemiluminescent detection system (ProteinSimple, USA).
Co-Immunoprecipitation
NK cells were lysed at room temperature for 30 min in 1 ml lysis buffer (25 mM Hepes (pH 7.4), 5 mM EDTA, 50 mM NaCl, 10% glycerol, 1% Triton X-100, 50 mM NaF, 30 mM sodium pyrophosphate and protease inhibitors (1 μg/ml each of aprotinin, leupeptin and pepstatin A, and 1 mM phenyl-methylsulfonyl fluoride)). The lysates were cleared by centrifugation at 12,000 g for10 min and protein content of the supernatant was evaluated using a BCA protein assay reagent kit (Yeasen, Shanghai). For co-immunoprecipitation, 500–1000 μg protein/ml was mixed with either 4 μg anti-HDAC3 antibody or 4 μg anti-STAT3 antibody (Santa Cruz Biotechnology) for 3 hrs. The antibody–antigen complexes were precipitated by protein G-linked Sepharose (Pharmacia) for 30 min and the beads were washed twice with lysis buffer. The denatured co-immunoprecipitation products were subjected to SDS-PAGE analysis, followed by immunoblotting using Enhanced Chemiluminescence Substrate (MerckMillipore, USA) according to the manufacturer’s instructions. Bands were visualized using a chemiluminescent detection system (ProteinSimple, USA).
Statistical analysis
Results are expressed as the mean ± standard error of the mean (S.E.M). One way ANOVA and independent samples t-test were used to measure statistical significance between the mean in all experiments. P-values of less than 0.05 were considered significant.
Additional Information
How to cite this article: Ni, L. et al. The histone deacetylase inhibitor valproic acid inhibits NKG2D expression in natural killer cells through suppression of STAT3 and HDAC3. Sci. Rep. 7, 45266; doi: 10.1038/srep45266 (2017).
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Lanier, L. L. NK cell recognition. Annu Rev Immunol 23, 225–274 (2005).
Guerra, N. et al. NKG2D-deficient mice are defective in tumor surveillance in models of spontaneous malignancy. Immunity 28, 571–580, doi: 10.1016/j.immuni.2008.02.016 (2008).
Morvan, M. G. & Lanier, L. L. NK cells and cancer: you can teach innate cells new tricks. Nat Rev Cancer 16, 7–19, doi: 10.1038/nrc.2015.5 (2015).
Pietra, G. et al. Melanoma cells inhibit natural killer cell function by modulating the expression of activating receptors and cytolytic activity. Cancer Res 72, 1407–1415, doi: 10.1158/0008-5472.CAN-11-2544 (2012).
Castriconi, R. et al. Transforming growth factor beta 1 inhibits expression of NKp30 and NKG2D receptors: consequences for the NK-mediated killing of dendritic cells. Proc Natl Acad Sci USA 100, 4120–4125, (2003).
Della Chiesa, M. et al. The tryptophan catabolite L-kynurenine inhibits the surface expression of NKp46- and NKG2D-activating receptors and regulates NK-cell function. Blood 108, 4118–4125, doi: 10.1182/blood-2006-03-006700 (2006).
Peserico, A. & Simone, C. Physical and functional HAT/HDAC interplay regulates protein acetylation balance. J Biomed Biotechnol 2011, 371832, doi: 10.1155/2011/371832 (2011).
Thiagalingam, S. et al. Histone deacetylases: unique players in shaping the epigenetic histone code. Ann N Y Acad Sci 983, 84–100 (2003).
Bolden, J. E., Peart, M. J. & Johnstone, R. W. Anticancer activities of histone deacetylase inhibitors. Nat Rev Drug Discov 5, 769–784 (2006).
Armeanu, S. et al. Natural killer cell-mediated lysis of hepatoma cells via specific induction of NKG2D ligands by the histone deacetylase inhibitor sodium valproate. Cancer Res 65, 6321–6329 (2005).
Skov, S. et al. Cancer cells become susceptible to natural killer cell killing after exposure to histone deacetylase inhibitors due to glycogen synthase kinase-3-dependent expression of MHC class I-related chain A and B. Cancer Res 65, 11136–11145 (2005).
Diermayr, S. et al. NKG2D ligand expression in AML increases in response to HDAC inhibitor valproic acid and contributes to allorecognition by NK-cell lines with single KIR-HLA class I specificities. Blood 111, 1428–1436, doi: 10.1182/blood-2007-07-101311 (2008).
Zhang, C., Wang, Y., Zhou, Z., Zhang, J. & Tian, Z. Sodium butyrate upregulates expression of NKG2D ligand MICA/B in HeLa and HepG2 cell lines and increases their susceptibility to NK lysis. Cancer Immunol Immunother 58, 1275–1285, doi: 10.1007/s00262-008-0645-8 (2009).
Shi, P. et al. Valproic acid sensitizes pancreatic cancer cells to natural killer cell-mediated lysis by upregulating MICA and MICB via the PI3K/Akt signaling pathway. BMC Cancer 14, 370, doi: 10.1186/1471-2407-14-370 (2014).
Zhu, S. et al. The narrow-spectrum HDAC inhibitor entinostat enhances NKG2D expression without NK cell toxicity, leading to enhanced recognition of cancer cells. Pharm Res 32, 779–792, doi: 10.1007/s11095-013-1231-0 (2015).
Ogbomo, H., Michaelis, M., Kreuter, J., Doerr, H. W. & Cinatl, J. Jr. Histone deacetylase inhibitors suppress natural killer cell cytolytic activity. FEBS Lett 581, 1317–1322, doi: 10.1016/j.febslet.2007.02.045 (2007).
Rossi, L. E. et al. Histone deacetylase inhibitors impair NK cell viability and effector functions through inhibition of activation and receptor expression. J Leukoc Biol 91, 321–331, doi: 10.1189/jlb.0711339 (2011).
Saito, A. et al. A synthetic inhibitor of histone deacetylase, MS-27-275, with marked in vivo antitumor activity against human tumors. Proc Natl Acad Sci USA 96, 4592–4597 (1999).
Gottlicher, M. et al. Valproic acid defines a novel class of HDAC inhibitors inducing differentiation of transformed cells. EMBO J 20, 6969–6978, doi: 10.1093/emboj/20.24.6969 (2001).
Alter, G., Malenfant, J. M. & Altfeld, M. CD107a as a functional marker for the identification of natural killer cell activity. J Immunol Methods 294, 15–22, doi: 10.1016/j.jim.2004.08.008 (2004).
Zhu, S. et al. Transcription of the activating receptor NKG2D in natural killer cells is regulated by STAT3 tyrosine phosphorylation. Blood 124, 403–411, doi: 10.1182/blood-2013-05-499707 (2014).
Leus, N. G. et al. HDAC 3-selective inhibitor RGFP966 demonstrates anti-inflammatory properties in RAW 264.7 macrophages and mouse precision-cut lung slices by attenuating NF-kappaB p65 transcriptional activity. Biochem Pharmacol 108, 58–74, doi: 10.1016/j.bcp.2016.03.010 (2016).
Wang, Z. et al. HDAC6 promotes cell proliferation and confers resistance to gefitinib in lung adenocarcinoma. Oncol Rep 36, 589–597, doi: 10.3892/or.2016.4811 (2016).
Balasubramanian, S. et al. A novel histone deacetylase 8 (HDAC8)-specific inhibitor PCI-34051 induces apoptosis in T-cell lymphomas. Leukemia 22, 1026–1034, doi: 10.1038/leu.2008.9 (2008).
Yu, H., Lee, H., Herrmann, A., Buettner, R. & Jove, R. Revisiting STAT3 signalling in cancer: new and unexpected biological functions. Nat Rev Cancer 14, 736–746, doi: 10.1038/nrc3818 (2014).
Ferguson, S. D., Srinivasan, V. M. & Heimberger, A. B. The role of STAT3 in tumor-mediated immune suppression. J Neurooncol 123, 385–394, doi: 10.1007/s11060-015-1731-3 (2015).
Wake, M. S. & Watson, C. J. STAT3 the oncogene - still eluding therapy? FEBS J 282, 2600–2611, doi: 10.1111/febs.13285 (2015).
Gupta, M., Han, J. J., Stenson, M., Wellik, L. & Witzig, T. E. Regulation of STAT3 by histone deacetylase-3 in diffuse large B-cell lymphoma: implications for therapy. Leukemia 26, 1356–1364, doi: 10.1038/leu.2011.340 (2012).
Hu, E. et al. Identification of novel isoform-selective inhibitors within class I histone deacetylases. J Pharmacol Exp Ther 307, 720–728 (2003).
Liu, X. et al. Identification of microRNA transcriptome involved in human natural killer cell activation. Immunol Lett 143, 208–217 (2012).
Panopoulos, A. D. et al. STAT3 governs distinct pathways in emergency granulopoiesis and mature neutrophils. Blood 108, 3682–3690, doi: 10.1182/blood-2006-02-003012 (2006).
Wang, X. et al. Membrane-bound interleukin-21 and CD137 ligand induce functional human natural killer cells from peripheral blood mononuclear cells through STAT-3 activation. Clin Exp Immunol 172, 104–112, doi: 10.1111/cei.12034 (2013).
Cholujova, D. et al. Comparative study of four fluorescent probes for evaluation of natural killer cell cytotoxicity assays. Immunobiology 213, 629–640, doi: 10.1016/j.imbio.2008.02.006 (2008).
Acknowledgements
This study was supported by National Natural Science Foundation of China (81473237, 81273216), the Shanghai Foundation for Development of Science and Technology (14431902600), the budget project of Shanghai Municipal Education Commission (2016YSN01) and the foundation for Shanghai municipal commission of health and family planning (20154Y0167).
Author information
Authors and Affiliations
Contributions
L.W. and L.N. performed the experiments and analyzed data. C.Y., Z.N., F.L. and X.Y. participated in the experiments. X.Z. carried out the N.K. cell expansion. C.G. performed the statistical analysis. S.S.W. contributed vital murine samples. D.A.L. and S.Z. conceived of the study, and participated in its design and coordination and wrote the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Ni, L., Wang, L., Yao, C. et al. The histone deacetylase inhibitor valproic acid inhibits NKG2D expression in natural killer cells through suppression of STAT3 and HDAC3. Sci Rep 7, 45266 (2017). https://doi.org/10.1038/srep45266
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep45266
This article is cited by
-
Impact of IL-15 and latency reversing agent combinations in the reactivation and NK cell-mediated suppression of the HIV reservoir
Scientific Reports (2022)
-
Valproic acid restricts mast cell activation by Listeria monocytogenes
Scientific Reports (2022)
-
Astragaloside IV suppresses post-ischemic natural killer cell infiltration and activation in the brain: involvement of histone deacetylase inhibition
Frontiers of Medicine (2021)
-
Combination chemotherapy of valproic acid (VPA) and gemcitabine regulates STAT3/Bmi1 pathway to differentially potentiate the motility of pancreatic cancer cells
Cell & Bioscience (2019)
-
Selective HDAC6 inhibitors improve anti-PD-1 immune checkpoint blockade therapy by decreasing the anti-inflammatory phenotype of macrophages and down-regulation of immunosuppressive proteins in tumor cells
Scientific Reports (2019)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.