Abstract
Dopmine functionalized mesoporous onion-like silica (DPMS) was synthesized via a biomimetic coating, and lipase Candida sp. 99-125 (LCS) was immobilized in DPMS (LCS@DPMS) by physical adsorption in this study. The DPMS was characterized by SEM, TEM, BET and FT-IR, and it was shown that the DPMS had clear multishell structures with large surface area of 419 m2/g. The activity, pH stability, thermal stability, storage stability, and reusability of the LCS@DPMS were investigated in detail. The stabilities of LCS@DPMS were improved significantly compared to the free lipase and LCS@MS (LCS immobilized in unfunctionalized mesoporous onion-like silica by physical adsorption). All the results indicated that the DPMS had high efficiency and improved stability for lipase immobilization.
Similar content being viewed by others
Introduction
Enzyme-catalyzed processes are widely used in the pharmaceutical or food industry due to their ease of production, green chemistry and substrate specificity1. The use of free enzymes is often hampered by several drawbacks such as low stability, high cost and poor reusability2. Immobilization of enzymes on solid supports is one of the best methods for biocatalysis to increase the stability and reusability of enzymes, simplify purification and recycling processes3,4. In the last few decades, numerous immobilization approaches and supports materials have been investigated5. In particular, mesoporous silica supports have attracted much attention due to their well-defined pore sizes and geometry, large specific surface area, and mechanical stability6,7. These advantages of mesoporous silica supports enable the improvement of activity and stability of enzymes. Recently, a novel meso-structured onion-like silica (Meso-Onion-S), which had highly curved mesopores and highly ordered onion-like multilayer, was synthesized and used as a host for the immobilization of enzyme, and the stability of the enzyme was effectively improved8,9. However, there are only two reports regarding the study of Meso-Onion-S, and therefore further studies for the immobilization of enzyme in this novel porous material are indeed required.
Typical strategies for immobilizing enzymes onto mesoporous silicas rely on surface grafting via polymers containing functional groups or low molecular weight linkers to which enzymes are reacted by covalent conjugation10,11,12. But such immobilization methods are usually accompanied by enzymes deactivation13. Thus, despite numerous reported approaches for immobilizing enzymes on mesoporous silicas, there is still the need for a simple, facile and cost-effective surface modification approach. Recently, inspired by the adhesive protein secreted by mussels, a mild approach of surface modification utilizing dopamine as functionalization reagent which can be simply attached onto almost all types of material surfaces, has been reported by Messersmith and co-workers14. Furthermore, the dopamine molecules contain both catechol and primary amine, which are capable of immobilizing biomolecules15,16. Therefore, immobilizing enzymes onto different substrates modified with dopamine can be easily achieved by facile conjugation of proteins12,15,16,17. However, to the best of our knowledge, Meso-Onion-S modified with dopamine has never been used as enzyme immobilization support. Ongoing efforts are necessary for the study on immobilization of enzyme in this new matrix.
Lipase is widely used in the food, fine chemical and pharmaceutical industries because of the versatile reactions it catalyses, such as hydrolysis, transesterification and esterification18,19,20,21. Lipase from Candida sp. 99-125 is an important enzyme with high activity and stability, and it is also cost-effective compared to other lipases22. Therefore, lipase Candida sp. 99-125 has great potential for applications in industry. However, to the best of our knowledge, there is no report for the immobilization of lipase Candida sp. 99-125 onto supports modified with dopamine so far. Thus, in this work, Meso-Onion-S was functionalized with dopamine (DPMS) via a biomimetic coating, and lipase Candida sp. 99-125 (LCS) was immobilized in DPMS (LCS@DPMS) by physical adsorption. This is the first time to explore the feasibility of immobilizing enzyme in Meso-Onion-S via a biomimetic coating. The activity, pH stability, thermal stability, storage stability, and reusability of the LCS@DPMS were investigated in detail. The results demonstrated that the DPMS exhibited high efficiency and improved stability for lipase immobilization, and it had great potential for practical applications.
Materials and methods
Materials
Poly (ethylene glycol)-b-poly (propylene glycol)-b-poly (ethylene glycol) (P123), tetraethoxysilane (TEOS) and dopamine·HCl were purchased from Sigma-Aldrich. 1,3,5-trimethylbenzene (TMB) was purchased from Meryer, China. Lipase Candida sp. 99-125 (the protein concentration in the crude lipase is 104.7 mg/g) was purchased from Beijing CTA New Century Biotechnology Co., Ltd., China. All other reagents were of AR grade.
Preparation of dopamine-functionalized Meso-Onion-S (DPMS)
The Meso-Onion-S was synthesized according to the method reported by Jun et al.8. Meso-Onion-S was functionalized with dopamine using the post-grafting method. Typically, 0.5 g of Meso-Onion-S was added into 100 mL of 1.25 g/L dopamine solution, which was freshly prepared in Tris-HCl buffer (pH 8.5), and the suspension was stirred for 2 h. Then, the solid was filtered and washed with phosphate buffer (pH 8.0) for three times. The as-prepared solid product was denoted as DPMS.
Immobilization of lipase in DPMS
Immobilization of lipase in DPMS was performed by physical adsorption method. 50 mg of DPMS was mixed with 5 mL of lipase solution with different concentrations (0.1 M phosphate buffer, pH 8.0) and the suspension was stirred with 200 rpm at 25 °C for 30 min. Then the mixture was centrifuged, washed with phosphate buffer (0.1 M, pH 8.0) for three times, freeze dried in a vacuum chamber, and stored in a fridge under −20 °C. The immobilized lipase was labelled as LCS@DPMS. The loading amount of lipase in DPMS was determined by the Bradford protein assay using bovine serum albumin as a standard. Furthermore, lipase Candida sp. 99-125 was also immobilized in Meso-Onion-S by physical adsorption (named LCS@MS) to compare it with that of LCS@DPMS.
Enzymatic activity assay
Enzymatic activities of native or immobilized lipase were assayed by the hydrolysis of 5 mg/mL 4-nitrophenyl palmitate (pNPP) in ethanol. Typically, 200 μL of pNPP was added into the enzyme solution (0.1 M phosphate buffer, pH 7.4), and the mixture was shaken at room temperature for 3 min20. After the reaction, the suspension was filtered and the concentration of the reaction product of p-nitrophenol (pNP) was measured at 405 nm. One unit of lipase activity was defined as that the quantity of enzyme which under the assay conditions liberates 1 μM of pNP per minute.
Determination of pH optima
In order to determine the optimum pH value, the free and immobilized lipase were incubated in 0.1 M phosphate buffer solutions with different pH values (5.0, 6.0, 7.0, 8.0 and 9.0) at 25 °C for 4 h, and the residual activities were measured. The excessive acidity and alkalinity of the phosphate buffer solutions were adjusted by 0.1 M HCl or 0.1 M NaOH. The relative activity was defined as the percentage of the residual activity compared to the initial activity.
Thermal and storage stability
The thermal stability was determined by immersing the free and immobilized lipase in isooctane at 60 °C and incubated for a certain period of time, and the residual activities were measured at each time point. The relative activities were calculated as described above.
To determine the storage stability, the free and immobilized lipase were immersed in 0.1 M phosphate buffer solutions with pH 8.0, and stored in a fridge under 4 °C. The residual activities were measured at each time point, and the relative activities were calculated.
Stability of free and immobilized lipase in shaking conditions
The free lipase, LCS@MS and LCS@DPMS were immersed in 0.1 M phosphate buffer solutions with pH 8.0 at room temperature and shaken at a speed of 200 rev/min. The residual activities were measured at each time point, and the relative activities were calculated.
Determination of kinetic parameters
The Michaelis-Menten kinetic parameters of free and immobilized lipase were determined by measuring the lipase activity using p-NPP as substrate in the initial concentration range from 1.32 to 13.2 μmol/L at 298 K and pH 8.0. The values of kinetic parameters Km and Vm were obtained by fitting the experimental data to the Michaelis-Menten model using non-linear regression code.
Reusability
The reusability of immobilized lipase was determined by conducting the enzymatic activity assay method described in activity assay section. At the end of each batch reaction, the immobilized lipase was recovered with centrifugation, washed excessively with buffer solution in order to remove the product or substrate and dried at room temperature. Then, the lipase was applied again in the next reaction cycle with fresh substrates.
Characterizations
SEM images of DPMS were obtained by a scanning electron microscopy (S-4800, Hitachi, Japan). TEM images were realized by a transmission electron microscopy (JEM-2100F, JEOL, Japan) operating at 200 kV. The surface-area measurements of Meso-Onion-S and DPMS were based on the Brunauer-Emmett-Teller method (BET, BELSORP-max, BEL, Japan), and the pore size distributions were obtained from adsorption isotherm branches by the Barrett-Joyner-Halenda (BJH) method. The Fourier transform infrared (FT-IR, VECTOR22, Bruker, Germany) spectra of Meso-Onion-S, DPMS and LCS@DPMS were recorded using the KBr pellet method.
Results and Discussion
Characterization of the immobilized lipase
The SEM photograph revealed that the DPMS was made up of hundreds of nanometer sized onions which aggregated into secondary micrometer sized particles (Fig. 1), and this result was in accordance with the previous reports8,9. The TEM image showed that the DPMS had clear multishell structures with mesopore size of about 13 nm between the layers (Fig. 2).
Figure 3 shows the nitrogen adsorption/desorption isotherms of Meso-Onion-S and DPMS. The specific surface area of DPMS was estimated as 419 m2/g, and the BJH adsorption cumulative volume and pore size were 1.14 cm3/g and 12.3 nm, respectively. Such pore size and pore volume were large enough to ensure the accommodation of enzymes. Compared with unfunctionalized Meso-Onion-S, the reduction of specific surface area, cumulative volume and pore size of DPMS indicated the success of modification.
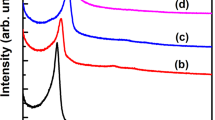
Figure 4 exhibits the FTIR spectra of Meso-Onion-S, DPMS and LCS@DPMS. Typical peaks at 467 cm−1, 796 cm−1 and 1086 cm−1 corresponded to the bending vibration, symmetric stretching and asymmetric stretching of Si-O-Si bonds from all of the samples, suggesting that the structure of Meso-Onion-S was well preserved in DPMS and LCS@DPMS23. Characteristic band at 962 cm−1 represented the symmetric stretching vibration of Si-OH groups24. The adsorption peak at 1495 cm−1 of DPMS was belong to the stretching of benzene ring in the polydopamine17, which indicated the successful immobilization of dopamine. For the LCS@DPMS, the peaks at 1545 cm−1 and 1465 cm−1 could be assigned to the deformation vibration of -NH2 groups and bending vibration of the -C-H groups2. This observation confirmed the successful immobilization of the lipase in DPMS.
Figures 5 and 6 shows the effect of lipase concentration on the lipase loading amount and activities of LCS@DPMS, respectively. According to the results, the lipase concentration was set at 10 mg/mL in the subsequent enzyme immobilization experiments. The results indicated that the lipase loading amount of LCS@DPMS was 81.6 mg/g carrier, and the immobilized yield was 52.4%. In the control experiment, the lipase was also immobilized in Meso-Onion-S by physical adsorption (named LCS@MS) under the same preparation conditions. However, the enzyme loading amount of LCS@MS was 62.2 mg/g carrier, and the immobilized yield was 45.3%, which was lower than that of LCS@DPMS. The improved lipase loading capacity and specific activity of LCS@DPMS could be attributed to the strong conjugation of covalent bond between the lipase molecules and the dopamine functionalized Meso-Onion-S, which might promote the lipase loading amount of LCS@DPMS.
Effect of pH on the activities of free and immobilized lipase
The effect of pH on the activities of free and immobilized lipase is shown in Fig. 7. The optimum pH for free lipase and LCS@MS was observed at 8.0, while LCS@DPMS reached its maximal activity at 7.0. Compared to the free lipase and LCS@MS, the optimum pH of LCS@DPMS exhibited a shift toward the acidic region. The polydopamine on the surface of DPMS contains quinones, which are reactive toward nucleophilic groups and can react with the amino or thiol groups of the lipase, and this reaction can lead to the immobilization of lipase in DPMS via covalent bond formation between the polydopamine and the lipase molecules16,25. The more acidic optimal pH of LCS@DPMS might be caused by the following two reasons: (i) the covalent attachment between the lipase and polydopamine on the surface of the support that limited the transition of lipase conformation against the change of pH12,26, and (ii) the amine or imines groups on the surface of polydopamine near the active sites of lipase molecules could diminish the concentration of protons15,17. Additionally, the pH profile of LCS@DPMS was wider range than free lipase and LCS@MS, which enhanced stability of LCS@DPMS under acidic conditions. Therefore, the LCS@DPMS was more suitable for widespread practical applications.
The thermal stability of free and immobilized lipase
Thermal stability is an important property of enzyme for industrial applications. The results of thermal stabilities of free lipase, LCS@MS and LCS@DPMS are shown in Fig. 8. Obviously, after 20 min of incubation at 60 °C, the free lipase and LCS@MS retained 54% and 65% of their original activities, respectively, while the remaining activity of LCS@DPMS was 73%. After 60 min the remaining activities of free lipase and LCS@MS decreased to 17% and 28%, respectively, and that of LCS@DPMS decreased to 41%. The half-life of lipase Candida sp. 99-125 at 60 °C was prolonged 3.31 times from 12.8 min (free lipase) to 42.4 min (LCS@DPMS). The multipoint interactions between the lipase and the polydopamine on the surface of DPMS could promoted the exposure of the catalytic domain of lipase molecules through conformational changes to the open lid domain, and then enhanced the adsorption of substrate molecules to the active sites27,28,29,30. Moreover, the covalent bonds between the lipase molecules and polydopamine could enhance the rigidity of lipase, and protected them from unfolding and then prevented their denaturation at high temperature31,32,33. For these reasons, the thermal stability of LCS@DPMS was meliorated comparing with the free lipase and LCS@MS.
The storage stability of free and immobilized lipase
The results of storage stability of the free and immobilized lipase are shown in Fig. 9. Evidently, the activity of the immobilized lipase decreased slowly than that of the free lipase. After 30 days, the relative activity of the LCS@DPMS could maintain 64%, whereas the relative activities of the free lipase and LCS@MS remained 16% and 42%, respectively. Thus, the LCS@DPMS exhibited higher storage stability than the free lipase and LCS@MS. The lipase was adsorbed in Meso-Onion-S via relatively weak physical force, and in contrast, the polydopamine on the surface of DPMS could react with the lipase molecules to form covalent conjugation, which could increase the conformational stability of lipase34,35. Therefore, the reason for the improved storage stability of LCS@DPMS might be that the LCS@DPMS could protect the active sites from deactivating more effectively under the same conditions compared with the free lipase and LCS@MS.
The stability of free and immobilized lipase in shaking conditions
The stability of the immobilized enzyme in shaking conditions is one of the important issues for most enzymatic reactions that are conducted in vigorous shaking for reducing mass transfer limitations2. Figure 10. shows the stability of free and immobilized lipase in shaking conditions (200 rev/min). It could be seen that the activity of the free lipase decreased fast and only 9.7% of the initial activity was retained the next day, and the reason for the decrease of activity was that the vigorous shaking could result in the autolysis and denaturation of the lipase9,36. Adsorption of the enzyme in Meso-Onion-S and DPMS improved the stability of lipase, and the LCS@MS retained 24% of its initial activity after shaking for 7 days, and in contrast, the LCS@DPMS retained 53% of its initial activity under the same conditions. The activity reduction was ascribed to the leaching of enzyme from the immobilized lipase37,38. Compared to the LCS@MS, the improved storage stability of LCS@DPMS might be ascribed to the strong conjugation between the lipase molecules and the polydopamine on the surface of Meso-Onion-S, which could prevent the lipase leaching effectively.
Kinetics of free and immobilized lipase
The kinetic parameters of the free lipase, LCS@MS and LCS@DPMS are listed in Table 1. The Km values of the LCS@MS and LCS@DPMS were 1.76 and 1.58 times higher than that of the free lipase, respectively, indicating that the substrate’s affinity capacity for the immobilized lipase was lower compared to the free lipase. The change in the substrate’s affinity for the lipase was probably caused by conformational change in the adsorption process of lipase onto supports, and resulting in a lower accessibility of the substrate toward the active sites of the immobilized lipase9,39. Furthermore, the Km value of the LCS@DPMS was lower than that of the LCS@MS, which might be ascribed to that the polydopamine on the surface of Meso-Onion-S could keep the conformation of the immobilized lipase more integrated and then enhance the affinity capacity between the lipase and the substrate7.
Reusability of the immobilised lipases
For practical applications, the reuse of immobilised lipase is necessary. The reusability of lipase immobilized in Meso-Onion-S and DPMS is shown in Fig. 11. It could be found that the LCS@MS and LCS@DPMS retained 63 and 71% of their initial activities after 6 consecutive operations, respectively. The decrease in activity was mainly caused by the denaturation and leaching of enzyme from the immobilised lipases35,40. Contrasting to LCS@MS, the activity of LCS@DPMS decreased slowly. The low operating stability of LCS@MS might result from that the lipase was adsorbed on Meso-Onion-S through physical force which was relatively weak and the lipase was easy to leach during the catalytic process34. The improved operational stability of LCS@DPMS was caused by the strong conjugation of covalent bond between the lipase molecules and the dopamine functionalized Meso-Onion-S, and thus the leakage of lipase from the DPMS was prevented. Moreover, the covalent bond could prevent the denaturation of lipase molecules upon use31,32,33. The more stable performance of LCS@DPMS in repeated use will make it desirable for practical applications.
Conclusions
In summary, a simple and cost-effective method was used to immobilize lipase Candida sp. 99-125 in mesoporous onion-like silica based on grafting polydopamine onto the silica, and the pH, thermal and storage stability of the immobilized lipase LCS@DPMS were improved significantly. Additionally, the pH profile of LCS@DPMS was wider range than free lipase and LCS@MS, and the performance of its reusability was more stable than LCS@MS. Taken together, the DPMS had great potential as a matrix for practical applications in the immobilization of lipase.
Additional Information
How to cite this article: Gao, J. et al. Dopamine-functionalized mesoporous onion-like silica as a new matrix for immobilization of lipase Candida sp. 99-125. Sci. Rep. 7, 40395; doi: 10.1038/srep40395 (2017).
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Hartmann, M. & Kostrov, X. Immobilization of enzymes on porous silicas–benefits and challenges. Chem. Soc. Rev. 42, 6277–6289 (2013).
Gao, J., Shi, L., Jiang, Y., Zhou, L. & He, Y. Formation of lipase Candida sp. 99-125 CLEAs in mesoporous silica: characterization and catalytic properties. Catal. Sci. Technol. 3, 3353 (2013).
Terentyeva, T. G. et al. Bioactive flake–shell capsules: soft silica nanoparticles for efficient enzyme immobilization. J. Mater. Chem. B 1, 3248 (2013).
Ling, D. et al. A general strategy for site-directed enzyme immobilization by using NiO nanoparticle decorated mesoporous silica. Chem. Eur. J. 20, 1–7 (2014).
Datta, S., Christena, L. R. & Rajaram, Y. R. S. Enzyme immobilization: an overview on techniques and support materials. 3 Biotech. 3, 1–9 (2012).
Hwang, E. T. et al. Immobilization and stabilization of subtilisin Carlsberg in magnetically-separable mesoporous silica for transesterification in an organic solvent. Green Chem. 14, 1884 (2012).
Bai, Y. X., Li, Y. F., Yang, Y. & Yi, L. X. Covalent immobilization of triacylglycerol lipase onto functionalized novel mesoporous silica supports. J. biotechnol. 125, 574–582 (2006).
Jun, S.-H. et al. Highly efficient enzyme immobilization and stabilization within meso-structured onion-like silica for biodiesel production. Chem. of Mater. 24, 924–929 (2012).
Gao, J. et al. Formation of Nitrile Hydratase cross-linked enzyme aggregates in mesoporous onion-like silica: preparation and catalytic properties. Ind. Eng. Chem. Res. 54, 83–90 (2015).
Kim, M. I. et al. Immobilization of Mucor javanicus lipase on effectively functionalized silica nanoparticles. J. Mol. Catal. B: Enzym. 39, 62–68 (2006).
Babaki, M., Yousefi, M., Habibi, Z., Brask, J. & Mohammadi, M. Preparation of highly reusable biocatalysts by immobilization of lipases on epoxy-functionalized silica for production of biodiesel from canola oil. Biochem. Eng. J. 101, 23–31 (2015).
Jiang, Y. et al. Facile immobilization of enzyme on three dimensionally ordered macroporous silica via a biomimetic coating. New J. Chem. 39, 978–984 (2015).
Lu, S., An, Z., He, J. & Li, B. Hierarchically-structured immobilized enzyme displaying the multi-functions of bio-membranes. J. Mater. Chem. 22, 3882 (2012).
Lee, H., Dellatore, S. M., Miller, W. M. & Messersmith, P. B. Mussel-inspired surface chemistry for multifunctional coatings. Science 318, 426–430 (2007).
Lee, H., Rho, J. & Messersmith, P. B. Facile Conjugation of Biomolecules onto Surfaces via Mussel Adhesive Protein Inspired Coatings. Adv. Mater. 21, 431–434 (2009).
Ren, Y. et al. Facile, high efficiency immobilization of lipase enzyme on magnetic iron oxide nanoparticles via a biomimetic coating. BMC Biotechnol., 11, 63 (2011).
Sureshkumar, M. & Lee, C.-K. Polydopamine coated magnetic-chitin (MCT) particles as a new matrix for enzyme immobilization. Carbohyd. Polym. 84, 775–780 (2011).
de Souza, R. L. et al. Protic ionic liquid as additive on lipase immobilization using silica sol-gel. Enzyme microb. tech. 52, 141–150 (2013).
Kharrat, N., Ali, Y. B., Marzouk, S., Gargouri, Y.-T. & Karra-Châabouni, M. Immobilization of Rhizopus oryzae lipase on silica aerogels by adsorption: comparison with the free enzyme. Process Biochem. 46, 1083–1089 (2011).
Kalantari, M., Kazemeini, M., Tabandeh, F. & Arpanaei, A. Lipase immobilisation on magnetic silica nanocomposite particles: effects of the silica structure on properties of the immobilised enzyme. J. Mater. Chem. 22, 8385 (2012).
Izrael Živković, L. T. et al. Immobilization of Candida rugosa lipase by adsorption onto biosafe meso/macroporous silica and zirconia. Biochem. Eng. J. 93, 73–83 (2015).
Lu, J., Nie, K., Wang, F. & Tan, T. Immobilized lipase Candida sp. 99-125 catalyzed methanolysis of glycerol trioleate: solvent effect. Bioresource technol. 99, 6070–6074 (2008).
Gao, J. K., Hou, L. A., Zhang, G. H. & Gu, P. Facile functionalized of SBA-15 via a biomimetic coating and its application in efficient removal of uranium ions from aqueous solution. J. Hazard. Mater. 286, 325–333 (2015).
Chen, Y., Gao, J., Wen, X. & Wu, W. Efficient removal of cadmium using facile functionalized of mesoporous silica via a biomimetic coating. RSC Adv. 6, 18340–18347 (2016).
Lee, H., Scherer, N. F. & Messersmith, P. B. Single-molecule mechanics of mussel adhesion. P. Natl. Acad. Sci. USA 103, 12999–13003 (2006).
Wu, J. C. et al. Enhanced enantioselectivity of immobilized Candida antarctica lipase for hydrolysis of ketoprofen ethyl ester at pH 1. Korean J. Chem. Eng. 24, 648–650 (2007).
Joyce, P., Whitby, C. P. & Prestidge, C. A. Interfacial processes that modulate the kinetics of lipase-mediated catalysis using porous silica host particles. RSC Adv. 6, 43802–43813 (2016).
Joyce, P., Kempson, I. & Prestidge, C. A. Orientating lipase molecules through surface chemical control for enhanced activity: a QCM-D and ToF-SIMS investigation. Colloid. surface. B 142, 173–181 (2016).
Reis, P. et al. Lipase-catalyzed reactions at different surfaces. Langmuir 22, 8169–8177 (2006).
Reis, P., Holmberg, K., Watzke, H., Leser, M. E. & Miller, R. Lipases at interfaces: a review. Adv. colloid interfac. 147–148, 237–250 (2009).
Yong, Y. et al. Characterization of Candida rugosa lipase immobilized onto magnetic microspheres with hydrophilicity. Process Biochem. 43, 1179–1185 (2008).
Iyer, P. V. & Ananthanarayan, L. Enzyme stability and stabilization—Aqueous and non-aqueous environment. Process Biochem. 43, 1019–1032 (2008).
Pizarro, C. et al. Influence of different immobilization techniques for Candida cylindracea lipase on its stability and fish oil hydrolysis. J. Mol. Catal. B: Enzym. 78, 111–118 (2012).
Wang, H., Jiang, Y., Zhou, L., He, Y. & Gao, J. Immobilization of penicillin G acylase on macrocellular heterogeneous silica-based monoliths. J. Mol. Catal. B: Enzym. 96, 1–5 (2013).
Chen, L., Wei, B., Zhang, X. & Li, C. Bifunctional graphene/gamma-Fe(2)O(3) hybrid aerogels with double nanocrystalline networks for enzyme immobilization. Small 9, 2331–2340 (2013).
Lee, J. et al. Magnetically-separable and highly-stable enzyme system based on crosslinked enzyme aggregates shipped in magnetite-coated mesoporous silica. J. Mater. Chem. 19, 7864 (2009).
Kim, M. I. et al. Crosslinked enzyme aggregates in hierarchically-ordered mesoporous silica: a simple and effective method for enzyme stabilization. Biotechnol. Bioeng. 96, 210–218 (2007).
Lee, J. et al. Simple synthesis of hierarchically ordered mesocellular mesoporous silica materials hosting crosslinked enzyme aggregates. Small 1, 744–753 (2005).
Li, Y. et al. Pore size of macroporous polystyrene microspheres affects lipase immobilization. J. Mol. Catal. B: Enzym. 66, 182–189 (2010).
Yiu, H. H. P., Wright, P. A. & Botting, N. P. Enzyme immobilisation using SBA-15 mesoporous molecular sieves with functionalised surfaces. J. Mol. Catal. B: Enzym. 15, 81–92 (2001).
Acknowledgements
The authors are grateful for financial supports by the National Natural Science Foundation of China (No. 51606168), National Sparking Plan Project (2013GA700254), Zhejiang Provincial Natural Science Foundation of China (Y3110170), National Training Programs of Innovation and Entrepreneurship for Undergraduates (201310340007), and International S&T Cooperation Program of China (2012DFA30600).
Author information
Authors and Affiliations
Contributions
J.K.G., Y.J.J., J.S.L. and Y.C. conceived and designed the experiments. J.K.G., J.J.D. and Y.C. carried out the experiments and wrote the manuscript text. J.K.G., Y.J.J., Z.H. and Y.C. analyzed the data. J.K.G., Y.J.J., J.S.L., Z.H., J.J.D. and Y.C. discussed the results and reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Gao, J., Jiang, Y., Lu, J. et al. Dopamine-functionalized mesoporous onion-like silica as a new matrix for immobilization of lipase Candida sp. 99-125. Sci Rep 7, 40395 (2017). https://doi.org/10.1038/srep40395
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep40395
This article is cited by
-
Lipase immobilized on MTMS-modified ceramic membrane for enhanced activity and stability
Journal of Materials Science (2023)
-
Immobilization of lipase on the modified magnetic diatomite earth for effective methyl esterification of isoamyl alcohol to synthesize banana flavor
3 Biotech (2020)
-
Novel Application of Magnetic Protein: Convenient One-Step Purification and Immobilization of Proteins
Scientific Reports (2017)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.