Abstract
Uremic pruritus (UP), also known as chronic kidney disease–associated pruritus, is a common and disabling symptom in patients undergoing maintenance hemodialysis (MHD). The pathogenesis of UP is multifactorial and poorly understood. Outdoor air pollution has well-known effects on the health of patients with allergic diseases through an inflammatory process. Air pollution–induced inflammation could occur in the skin and aggravate skin symptoms such as pruritus or impair epidermal barrier function. To assess the role of air pollutants, and other clinical variables on uremic pruritus (UP) in HD patients, we recruited 866 patients on maintenance HD. We analyzed the following variables for association with UP: average previous 12-month and 24-month background concentrations for nitrogen dioxide (NO2) and carbon monoxide (CO), and suspended particulate matter of <2.5 μm (PM2.5). In a multivariate logistic regression, hemodialysis duration, serum ferritin levels, low-density lipoprotein levels, and environmental NO2/CO levels were positively associated with UP, and serum albumin levels were negatively associated with UP. This cross-sectional study showed that air pollutants such as NO2 and CO might be associated with UP in patients with MHD.
Similar content being viewed by others
Introduction
Uremic pruritus (UP), also known as chronic kidney disease–associated pruritus, is a common and disabling symptom in patients undergoing hemodialysis. About 42–90% of patients on dialysis experience pruritus1,2,3. This symptom lasts for months to years in most cases and is resistant to conventional treatments4. According to Mettang5, as patients with end-stage renal disease also have many other chronic diseases, definitive diagnosis of UP may be challenging. Until now, the pathogenesis of UP was considered multifactorial and still poorly understood6,7,8,9. At least 4 main hypotheses have been described, including dermatological abnormalities, an immune-system derangement that results in a proinflammatory state, an imbalance of the endogenous opioidergic system, and a neuropathic mechanism10.
Outdoor air pollution has well-known effects on the health of patients with allergic diseases. Exposure to air pollutants is related to neutrophilic inflammation and an increase in cytokine and IgE levels11,12,13, which aggravate dermatitis14. In urban areas, air particulates that are enriched with metals have also been believed to contribute to inflammatory responses15. Our previous study16 showed that residential area was a risk factor for 2-year mortality in patients undergoing hemodialysis, and mean concentration levels of air pollutants (NO2, CO and PM2.5) were significantly high in basin terrain. We also determined an association among air pollutants and mortality, inflammation, vascular disease, and infection in patients on dialysis17,18,19,20. Air pollution–induced inflammation could occur in the skin and aggravate skin symptoms such as pruritus or impair the epidermal barrier function21,22,23. However, to our knowledge, no in-depth study on the correlation between air pollutants and UP in maintenance hemodialysis (MHD) patients has been reported. The aim of this cross-sectional study was to assess the role of residential area, air pollutants, age, male sex, body mass index (BMI), smoking status, diabetes mellitus (DM), hypertension, previous cardiovascular diseases (CVDs), hepatitis B virus (HBV) infection, hepatitis C virus (HCV) infection, HD duration, blood access as arterial-venous(AV) fistula, undergoing hemodialfiltration (HDF), clearance of urea (Kt/Vurea Daugirdes), normalized protein catabolism rate (nPCR), non-anuria status, hemoglobin levels, serum albumin levels, serum creatinine levels, corrected calcium levels, inorganic phosphate levels, log-transformed (log) ferritin levels, log intact parathyroid hormone levels (log iPTH), log high-sensitivity C-reactive protein (log hsCRP) levels, low-density lipoprotein (LDL) levels and triglyceride levels on UP in hemodialysis (HD) patients.
Results
Finally, 866 MHD patients (440 men and 426 women) with a mean MHD duration of 6.96 ± 5.35 y were included in the study. Table 1 lists patient characteristics including age, gender, and BMI, along with the biological, hematological, and HD data for all patients and patients with or without UP. Among the patients, 50.8% were male, 21.8% had UP, 22.2% had a history of DM, 4.7% had CVDs, 2.9% had lupus, 17.3% were habitual users of tobacco, 79.6% had an AV fistula that was utilized, 11.3% had hepatitis B virus infection, and 19.4% had HCV infection.
Comparison of Clinical Variables between Patients with and without UP
Comparing patients with and without UP, a higher proportion of patients with UP lived in Taipei Basin (65.1% vs 6.6%, respectively; P < 0.001), had an HCV infection (24.9% vs 17.9%, respectively; P = 0.037), and had undergone HDF (27.5% vs 19.9%, respectively; P = 0.028), while a lower proportion had DM (13.2% vs 24.7%, respectively; P = 0.001) and a non-anuria condition (11.6% vs 23%; P < 0.001). Furthermore, patients with UP had longer HD durations (9.41 ± 5.43 vs 6.27 ± 5.13 y, respectively; P < .001), were older (57.93 ± 12.43 vs 55.70 ± 13.86 y, respectively; P = 0.047), had higher Kt/V Daugirdes (1.89 ± 0.34 vs 1.77 ± 0.32, respectively; P < 0.001), had higher nPCR (1.22 ± 0.27 vs 1.17 ± 0.26 g/kg/day, respectively; P = 0.03), had higher iPTH levels (187.2 vs 121.3 pg/mL, respectively; P = 0.003), had higher cholesterol levels (176.93 ± 39.24 vs 169.73 ± 37.08 mg/dL, respectively; P = 0.02), had higher LDL levels (100.36 ± 31.05 vs 93.26 ± 30.30 mg/dL, respectively; P = 0.005), and had lower serum albumin levels (4.01 ± 0.33 vs 4.07 ± 0.34 g/dL, respectively; P = 0.019) than patients without UP (Table 1).
Associations among UP, Clinical Variables, and Residential Area
Univariate logistic regression identified several clinical variables that were significantly associated with UP (Table 2), such as BMI, DM, HCV infection, HD duration, undergoing HDF, Kt/Vurea, nPCR, non-anuria status, serum albumin levels, log iPTH levels, LDL levels, living in Taipei Basin, environmental NO2 levels, and environmental CO levels. To determine the association between residential areas and UP, multivariate forward logistic regression analyses of variables with a P of <0.1 in the univariate logistic regression (excluding environmental NO2 and CO levels) indicated that HD duration (OR, 1.1; 95% CI, 1.05–1.14; P < 0.001), non-anuria status (OR, 0.30; 95% CI, 0.15–0.59; P < 0.001), log ferritin levels (OR, 1.65; 95% CI, 1.02–2.69; P = 0.043), LDL levels (OR, 1.01; 95% CI, 1.01–1.02; P < 0.001), and living in Taipei Basin (OR, 32.93; 95% CI, 20.14–53.83; P < 0.001) were associated with UP (Table 3).
Comparison of Clinical Variables between Patients Living in and around Taipei Basin
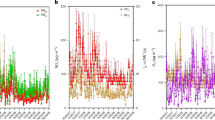
Patients living in Taipei Basin demonstrated a lower proportion of DM (13.1% vs 24.4%, respectively; P = 0.001) but demonstrated a higher proportion of UP (73.2% vs 9.5%, respectively; P < 0.001) and HDF use (27.4% vs 20.2%, respectively; P = 0.047) than patients living around Taipei Basin. Furthermore, patients living in Taipei Basin had a longer HD duration (8.71 ± 5.62 vs 6.54 ± 5.20 y, respectively; P < 0.001), higher Kt/V Daugirdes (1.88 ± 0.36 vs 1.77 ± 0.31, respectively; P < 0.001), higher nPCR (1.25 ± 0.27 vs 1.17 ± 0.26 g/kg/day, respectively; P < 0.001), higher iPTH levels (172.20 vs 123.40 pg/ml, respectively; P = 0.016), higher previous 12-month environmental NO2 levels (24.37 ± 6.47 vs 20.46 ± 5.57 ppb, respectively; P < 0.001), higher previous 12-month environmental CO levels (0.75 ± 0.38 vs 0.59 ± 0.29 ppm, respectively; P < 0.001), and higher previous 12-month environmental PM2.5 levels (29.60 ± 4.07 vs 28.32 ± 3.52 μg/m3, respectively; P < 0.001) than patients living around Taipei Basin (Table 4).
Associations among UP, Clinical Variables, and Environmental Air Pollutants (NO2 and CO)
To further investigate the association between environmental air pollutants and UP, we used a multivariate logistic regression analysis to evaluate the association between mean previous 12-month and 24-month air pollutant levels (NO2 and CO) and UP in the patients. The result indicated that after adjusting for related factors (including BMI, HD durations, DM, HBV, HCV, HDF, Kt/Vurea, nPCR, serum albumin levels, non-Anuria, corrected-calcium, Log iPTH, log ferritine, LDL levels, and mean previous 12- and 24-month Environmental PM2.5 level), UP in studied patients was positively associated with the mean previous 12-month environmental CO levels (OR, 1.73; 95% CI, 1.05–2.83; P = 0.03) and NO2 levels (OR, 1.05; 95% CI, 1.01–1.07; P = 0.001) levels (Table 5) and mean previous 24-month CO levels (OR, 1.82; 95% CI, 1.09–3.02; P = 0.02) and NO2 levels (OR, 1.05; 95% CI, 1.02–1.08; P = 0.001) (Table 6).
Discussion
The present cross-sectional study indicates that environmental NO2 and CO levels are significant factors for UP in patients undergoing HD after adjusting for related factors. We also found that the residential environment is a factor associated with UP that is consistent with our previous study on the importance of living environment in dialysis patients16.
The mechanism of UP is not yet fully understood. A pro-inflammatory state secondary to immune system derangement with a high level of some cytokines, opioid-receptor system abnormalities, malnutrition, and dermal mast cells interfacing with the distal ends of unmyelinated C fibers, as well as possible mineral bone metabolism and nutritional disturbances, are among various mechanisms considered related to its causality or intensity24,25,26. Pruritus in MHD patients might occur possibly because skin barrier destruction induces an increase in neuron-specific enolase-immunoreactive nerve fibers in the epidermis27. It is well known that outdoor air pollution affects the health of patients with allergic diseases28,29,30,31. To our knowledge, this study is the first to show that environmental air pollutants (NO2 and CO) are positively associated with UP in MHD patients. Several studies have shown associations between air pollution and skin allergic disease through an inflammatory response14,15,32,33,34. The role of air pollutants on UP is unclear. It is likely that air pollutants aggravate skin symptoms such as pruritus, possibly by inducing oxidative stress in the skin that leads to skin barrier dysfunction and inducing an increase in neuron fibers in the epidermis, immune dysregulation, or neurogenic inflammation by environmental air pollutions21,22,23,35,36.
As for the studied air pollutants, NO2, an oxidant pollutant, induces oxidative damage to cell membranes, resulting in the generation of reactive oxygen species and subsequent inflammation, and could affect immunoglobulin levels, complement levels, and T-cell functions37,38,39,40. In a large cohort study, Morgenstern et al. demonstrated that the appearance of eczema in children is associated with environmental NO2 and other traffic-related air pollutant exposure levels41. Kim et al. also indicated that environmental NO2 concentrations and other air pollutant levels were associated with atopic dermatitis14.
Discussions on the association between environmental CO exposure and pruritus are rare. In a nationwide survery in 6- to 7-year-old children, Kim et al.30 presented that previous 12-month CO exposure levels were with high odds ratio for the presence of atopic dermatitis. The relationship between environmental CO and dermatits is not clear. However, environmental CO levels were associated with hsCRP levels in patients undergoing peritoneal dialysis18. In other studies, intermittent CO exposure caused damage to arterial walls and resulted in atherosclerosis42,43,44. A study by Davutoglu et al. demonstrated these cardiovascular effects and showed that hsCRP levels and carotid intima–media thickness were increased in subjects with chronic CO exposure43. Previously cited studies and our findings indicated that the role of environmental CO exposure in MHD patients may be explained by the relationship between inflammation and worsening pruritus9,45,46. In addition, in Kim’s study, high odds ratio (8.11) of atopic dermatitis was noted as 1 ppm increased CO level30. It is not clear why the difference of odds ratio between Kim’s study and ours. The reasonable explanation for above difference could be the different studied patients, underling diseases, age and living habits.
Interestingly, the odds ratio for patients with UP living in Taipei basin was about 32.93 (95% CI, 20.14–53.83); however, for environmental air pollutants (NO2 and CO), the odds ratio was around 1.8 for environmental CO levels and 1.05 for environmental NO2 levels in UP. In our study, the air pollutants levels for NO2, CO and PM2.5 in Taipei Basin were higher than those around Taipei Basin. To our knowledge, there are several environmental factors including season, temperature, humidity, indoor pollutants levels, foods, contact irritants, and air pollutants levels that are associated with allergic disease or allergic sensitization14,15,21,32,34. In our study, the environmental PM2.5 level was positively associated with UP, but without significance (P > 0.05). A reasonable explanation might be that in this study, we did not include all environmental allergic factors (unavailable) such as other pollutants, humidity, season and temperature, and it seems likely that other environmental factors that we did not include in this study might be correlated with UP. From above, we also could explain why the large gaps in the odds ratio between residential area and air pollutants (CO and NO2). Although this study was designed as a cross-sectional study, we used the average previous 12-month and 24-month air pollutant levels including CO, NO2, and PM2.5 for our analysis of UP that seems to be similar to a semi-cohort–designed study. Our study suggested that chronic exposure to environmental air pollutants (NO2 and CO) might be associated with pruritus in patients with MHD.
In this study, HD duration is a factor associated with UP that is consistent with several studies47,48. However, a study by Malekmakan did not demonstrate this result45. In our study, patients who lived in Taipei Basin had a longer HD duration than those who lived around Taipei Basin. From discussion of previous study16, comparison of living environment between city and suburb situations is complex. Air pollution is the one factor that could be quantified efficiently. Exactly, it is the matter of international concern. The Taipei basin is surrounded by hills and mountains, and about 6 million people living in it. So, the complexity of the terrain increases the difficulty of the diffusion of air pollution. From above, long-term contact with inflammation-inducing substances such as environmental air pollutants (NO2 and CO) could explain the prevalence of UP in patients living in different areas.
This study had some limitations. First, we did not include other air pollutants and other environmental factors such as temperature, humidity, seasons, diets, mite or contract irritants. It means that we could have under- or overestimated the correlation between environmental air pollutants (NO2 and CO) and UP in this study. We also know that UP is difficult to treat and study on pruritus of HD patients is complicated because of above mentioned factors. In the treatment of UP, in our dialysis centers, we also offer many methods to avoid pruritus that we know. Second, this was a cross-sectional study, and we only found the correlation between environmental CO/NO2 and UP, not the relationship between cause and effect. Third, air pollution data were collected and calculated from the previous 12 months and 24 months. In several studies on the association between air pollution and allergic disease, the duration of observation is 12-month28,29,30,31. In this study, use of previous 24-month air pollutants levels for analysis additionally is consistent with the effects of previous 12-month environmental air pollutants levels.
Conclusion
This cross-sectional study showed that environmental air pollutants (NO2 and CO) might be a factor associated with UP in patients on MHD. Further, prevalence of UP may differ based on location. Further studies are required to clarify the role of other air pollutants or environmental factors on UP in patients undergoing MHD.
Patients and Methods
Methods
The Institutional Review Board Committee of Chang Gung Memorial Hospital approved the study protocol. Informed consent was obtained from all patients. All medical records during the study period, including medical history, laboratory data, and inclusion and exclusion factors, were reviewed by senior nephrologists. In addition, all individual information was securely protected and only available to the investigators. And all experiments protocols were conducted according to the Strengthening the Reporting of Observational Studies in Epidemiology guidelines.
Patients
This is a random-selection, cross-sectional study on patients undergoing MHD in 3 hemodialysis centers. Recruitment started in February 2013, and follow-up ended in December 2013. Study patients were recruited from the 3 hemodialysis centers of Chang Gung Memorial Hospital, Lin-Kou Medical Center at both the Taipei and Taoyuan branches. Only MHD patients who were 18 years of age or older and had undergone HD for at least 6 months were enrolled in this study. Residential areas were divided as follows: in Taipei Basin and around Taipei Basin. Coverage of Taipei Basin included: East, Nankang District of Taipei City; West, Shulin District of New Taipei City; South, Jingmei District of Taipei City; and North, Beitou District of Taipei City16. To be included in this study, patients had to be undergoing hemodialfiltration (HDF) 3 times weekly for ≥3 months. Patients with malignancies or obvious infectious diseases, as well as those who had been hospitalized or had undergone surgery within 3 months of the investigation, were excluded (Fig. 1). Diabetes mellitus (DM) was defined by either a physician’s diagnosis and antidiabetic drug treatment or 2 subsequent analyses demonstrated fasting blood glucose levels of >126 mg/dL. Most patients (98.2%) underwent 4 h of HD 3 times a week. HD was performed using single-use hollow-fiber dialyzers equipped with modified cellulose, polyamide, or polysulfone membranes. The dialysate used in all cases had a standard ionic composition with a bicarbonate-based buffer. We noted the incidence of cardiovascular diseases (CVDs) including cerebrovascular disease, coronary artery disease, congestive heart failure, and peripheral vascular disease in these patients. Hypertension was defined as the regular use of antihypertensive drugs to control blood pressure or at least 2 blood pressure measurements of more than 140/90 mmHg. Smoking behavior was also noted in this study. Diagnosis of pruritus was as follows: pruritus appearing after HD with/without antipruritics as visualized by trained dermatologists or nephrologists.
There are several air pollutants which are monitored for air quality control such as particulate matter with aerodynamic diameter less than 10 and 2.5 mm (PM10 and PM2.5), ozone (O3), sulfur dioxide (SO2), nitrogen dioxide (NO2) and carbon monoxide (CO). From our previous studies17,18,19,20, we found the association between environmental CO levels and inflammation in peritoneal dialysis (PD) patients, between environmental NO2 levels and mortality in PD patients, and between environmental particulate matter and PD-related infections in PD patients and arterial sclerosis in HD patients; therefore, we analyzed the association of above mentioned air pollutants (environmental NO2, CO, and PM2.5) levels in UP. Individual exposure to air pollution was estimated by a geographic information system using the mean previous 12- and 24-month concentrations of air pollutants.
Laboratory, Nutritional, and Inflammatory Parameters
All blood samples were drawn from the arterial end of the vascular access immediately after the initial 2-day interval for HD and were then centrifuged and stored at −80 °C until use. Serum creatinine levels, normalized protein catabolism rate (nPCR), and serum albumin levels were assayed and recorded as nutritional markers. High-sensitivity C-reactive protein (hsCRP) levels were measured as indices of inflammation. The serum hsCRP concentration was measured using immunonephelometry (Nanopia CRP; Daiichi Inc, Tokyo, Japan). The lowest detection limit was <0.15 mg/L. All other biochemical indices were measured using a standard laboratory approach with an automatic analyzer. In HD patients, dialyzer clearance of urea was measured using a method described by Daugirdas and was expressed as Kt/V urea49. The nPCR of the HD patients was calculated using validated equations and was normalized to their body weight50. The serum calcium level was corrected using the serum albumin level with the following formula: corrected calcium level (mg/dL) = serum calcium level + 0.8 × (4.0 − serum albumin level). Non-anuria was defined as a daily urine amount of ≥100 mL.
Statistical Analysis
The Kolmogorov–Smirnov test was used to test if variables were normally distributed. A P value of >0.05 was required to assume a normal distribution. Continuous variables are expressed as mean ± standard deviation/median (interquartile range), and categorical variables are expressed as numbers or percentages. X2 or Fisher’s exact test was used to analyze the correlation between categorical variables. Comparisons between 2 groups were performed using the Mann–Whitney U test or Student’s t test. Data involving hsCRP, intact parathyroid hormone, and ferritin levels were log-transformed for analysis. To evaluate the variables related to UP, univariate and multivariate (forward method) logistic regression analyses were performed to assess the odds ratio (OR) and 95% confidence interval (CI) for baseline variables including age, male sex, BMI, smoking status, DM, hypertension, previous CVDs, HBV infection, HCV infection, HD duration, blood access fistula, HDF, Kt/Vurea Daugirdes, nPCR, non-anuria status, hemoglobin levels, serum albumin levels, serum creatinine levels, corrected calcium levels, inorganic phosphate levels, log ferritin levels, log iPTH levels, log hsCRP levels, LDL levels, triglyceride levels, environmental NO2 levels (or environmental CO levels or environmental PM2.5 levels) (variables with a P value of <0.1 in the univariate logistic regression were selected for the multivariate logistic regression). According to the high collinearity (variance inflation factor [VIF]; NO2, 11.267; CO, 10.708) and high correlation between the environmental CO and NO2 levels, in the multivariate logistic regression, we included the 2 abovementioned items separately for the multivariate logistic regression analysis. All nominal variables in the logistic regression were transformed into dummy coding. Missing data were approached using list-wise deletion. Data were analyzed using SPSS, version 12.0 for Windows 95 (SPSS Inc, Chicago, IL). The level of significance was set at a P of <0.05.
Additional Information
How to cite this article: Huang, W.-H. et al. Environmental NO2 and CO Exposure: Ignored Factors Associated with Uremic Pruritus in Patients Undergoing Hemodialysis. Sci. Rep. 6, 31168; doi: 10.1038/srep31168 (2016).
References
Zucker, I., Yosipovitch, G., David, M., Gafter, U. & Boner, G. Prevalence and characterization of uremic pruritus in patients undergoing hemodialysis: uremic pruritus is still a major problem for patients with end-stage renal disease. J Am Acad Dermatol 49, 842–846 (2003).
Kimata, N. et al. Pruritus in hemodialysis patients: Results from the Japanese Dialysis Outcomes and Practice Patterns Study (JDOPPS). Hemodial Int 18, 657–667 (2014).
Pisoni, R. L. et al. Pruritus in haemodialysis patients: International results from the Dialysis Outcomes and Practice Patterns Study (DOPPS). Nephrol Dial Transplant 21, 3495–3505 (2006).
Mathur, V. S. et al. A longitudinal study of uremic pruritus in hemodialysis patients. Clin J Am Soc Nephrol 5, 1410–1419 (2010).
Mettang, T. & Kremer, A. E. Uremic pruritus. Kidney Int 87, 685–691 (2015).
Marquez, D. et al. Uremic pruritus in hemodialysis patients: treatment with desloratidine versus gabapentin. J Bras Nefrol 34, 148–152 (2012).
Murphy, M., Reaich, D., Pai, P., Finn, P. & Carmichael, A. J. A randomized, placebo-controlled, double-blind trial of ondansetron in renal itch. Br J Dermatol 148, 314–317 (2003).
Silva, S. R. et al. Thalidomide for the treatment of uremic pruritus: a crossover randomized double-blind trial. Nephron 67, 270–273 (1994).
Chiu, Y. L. et al. Association of uraemic pruritus with inflammation and hepatitis infection in haemodialysis patients. Nephrol Dial Transplant 23, 3685–3689 (2008).
Aucella, F. & Gesuete, A. Uremic pruritus: an unresolved challenge. G Ital Nefrol 26, 585–599 (2009).
McCreanor, J. et al. Respiratory effects of exposure to diesel traffic in persons with asthma. N Engl J Med 357, 2348–2358 (2007).
Diaz-Sanchez, D. The role of diesel exhaust particles and their associated polyaromatic hydrocarbons in the induction of allergic airway disease. Allergy 52, 52–56; discussion 57–58 (1997).
Nikasinovic, L. et al. Nasal inflammation and personal exposure to fine particles PM2.5 in asthmatic children. J Allergy Clin Immunol 117, 1382–1388 (2006).
Kim, J. et al. Symptoms of atopic dermatitis are influenced by outdoor air pollution. J Allergy Clin Immunol 132, 495–498 e491 (2013).
Happo, M. S. et al. Inflammation and tissue damage in mouse lung by single and repeated dosing of urban air coarse and fine particles collected from six European cities. Inhal Toxicol 22, 402–416 (2010).
Huang, W. H. et al. Impact of living environment on 2-year mortality in elderly maintenance hemodialysis patients. PLoS One 8, e74358 (2013).
Lin, J. H., Yen, T. H., Weng, C. H. & Huang, W. H. Environmental NO2 level is associated with 2-year mortality in patients undergoing peritoneal dialysis. Medicine (Baltimore) 94, e368 (2015).
Huang, W. H., Yen, T. H., Chan, M. J. & Su, Y. J. Environmental carbon monoxide level is associated with the level of high-sensitivity C-reactive protein in peritoneal dialysis patients. Medicine (Baltimore) 93, e181 (2014).
Weng, C. H., Hu, C. C., Yen, T. H. & Huang, W. H. Association between environmental particulate matter and arterial stiffness in patients undergoing hemodialysis. BMC Cardiovasc Disord 15, 115 (2015).
Huang, W. H., Yen, T. H., Chan, M. J. & Su, Y. J. Impact of environmental particulate matter and peritoneal dialysis-related infection in patients undergoing peritoneal dialysis. Medicine (Baltimore) 93, e149 (2014).
Song, S. et al. Acute health effects of urban fine and ultrafine particles on children with atopic dermatitis. Environ Res 111, 394–399 (2011).
Eberlein-Konig, B. et al. Influence of airborne nitrogen dioxide or formaldehyde on parameters of skin function and cellular activation in patients with atopic eczema and control subjects. J Allergy Clin Immunol 101, 141–143 (1998).
Huss-Marp, J. et al. Influence of short-term exposure to airborne Der p 1 and volatile organic compounds on skin barrier function and dermal blood flow in patients with atopic eczema and healthy individuals. Clin Exp Allergy 36, 338–345 (2006).
Nordal, E. J. & Os, I. Uremic pruritus–pathogenesis and treatment. Tidsskr Nor Laegeforen 127, 1201–1203 (2007).
Mettang, M. & Weisshaar, E. Pruritus: control of itch in patients undergoing dialysis. Skin Therapy Lett 15, 1–5 (2010).
Lugon, J. R. Uremic pruritus: a review. Hemodial Int 9, 180–188 (2005).
Johansson, O., Hilliges, M. & Stahle-Backdahl, M. Intraepidermal neuron-specific enolase (NSE)-immunoreactive nerve fibres: evidence for sprouting in uremic patients on maintenance hemodialysis. Neurosci Lett 99, 281–286 (1989).
Dunlop, J., Matsui, E. & Sharma, H. P. Allergic Rhinitis: Environmental Determinants. Immunol Allergy Clin North Am 36, 367–377 (2016).
Kim, H. H. et al. Near-Road Exposure and Impact of Air Pollution on Allergic Diseases in Elementary School Children: A Cross-Sectional Study. Yonsei Med J 57, 698–713 (2016).
Kim, J. et al. Association of carbon monoxide levels with allergic diseases in children. Allergy Asthma Proc 37, 1–7 (2016).
Lindgren, A., Stroh, E., Nihlen, U., Montnemery, P., Axmon, A. & Jakobsson, K. Traffic exposure associated with allergic asthma and allergic rhinitis in adults. A cross-sectional study in southern Sweden. Int J Health Geogr 8, 25 (2009).
Lee, Y. L., Su, H. J., Sheu, H. M., Yu, H. S. & Guo, Y. L. Traffic-related air pollution, climate, and prevalence of eczema in Taiwanese school children. J Invest Dermatol 128, 2412–2420 (2008).
Kramer, U. et al. Eczema, respiratory allergies, and traffic-related air pollution in birth cohorts from small-town areas. J Dermatol Sci 56, 99–105 (2009).
Lee, J. H. et al. The clinical effects of hospitalization in a low pollutant room on atopic dermatitis. Asia Pac Allergy 1, 87–92 (2011).
Ahn, K. The role of air pollutants in atopic dermatitis. J Allergy Clin Immunol 134, 993–1000 (2014).
Meggs, W. J. Neurogenic inflammation and sensitivity to environmental chemicals. Environ Health Perspect 101, 234–238 (1993).
Brook, R. D. et al. Particulate matter air pollution and cardiovascular disease: An update to the scientific statement from the American Heart Association. Circulation 121, 2331–2378 (2010).
Bind, M. A. et al. Air pollution and markers of coagulation, inflammation, and endothelial function: associations and epigene-environment interactions in an elderly cohort. Epidemiology 23, 332–340 (2012).
Moreno Ramiez, E., Hernandez Urzua, M. A., Gonzalez Villegas, A. C., Casas Solis, J. & Zaitseva, G. Study of immunoglobulins, proinflammatory cytokines, lymphoproliferation and phagocytosis in peripheral blood of healthy young people exposed to different levels of atmospheric pollution. Rev Alerg Mex 53, 3–8 (2006).
Rutowski, J., Moszczynski, P., Dobrowolski, J. W., Bem, S. & Krochmal, D. The effects of occupational exposure to nitrogen dioxide (NO2) on the immunological parameters in workers. Med Pr 49, 341–351 (1998).
Morgenstern, V., Zutavern, A., Cyrys, J. et al. Atopic diseases, allergic sensitization, and exposure to traffic-related air pollution in children. Am J Respir Crit Care Med 177, 1331–1337 (2008).
Astrup, P., Kjeldsen, K. & Wanstrup, J. Enhancing influence of carbon monoxide on the development of atheromatosis in cholesterol-fed rabbits. J Atheroscler Res 7, 343–354 (1967).
Davutoglu, V. et al. Chronic carbon monoxide exposure is associated with the increases in carotid intima-media thickness and C-reactive protein level. Tohoku J Exp Med 219, 201–206 (2009).
Smith, C. J. & Steichen, T. J. The atherogenic potential of carbon monoxide. Atherosclerosis 99, 137–149 (1993).
Malekmakan, L. et al. Association of high-sensitive C-reactive protein and dialysis adequacy with uremic pruritus. Saudi J Kidney Dis Transpl 26, 890–895 (2015).
Kimmel, M. et al. The role of micro-inflammation in the pathogenesis of uraemic pruritus in haemodialysis patients. Nephrol Dial Transplant 21, 749–755 (2006).
Szepietowski, J. C. et al. Uremic pruritus: a clinical study of maintenance hemodialysis patients. J Dermatol 29, 621–627 (2002).
Gatmiri, S. M., Mahdavi-Mazdeh, M., Lessan-Pezeshki, M. & Abbasi, M. Uremic pruritus and serum phosphorus level. Acta Med Iran 51, 477–481 (2013).
Daugirdas, J. T. The post: pre-dialysis plasma urea nitrogen ratio to estimate K.t/V and NPCR: mathematical modeling. Int J Artif Organs 12, 411–419 (1989).
Sargent, J. A. Control of dialysis by a single-pool urea model: the National Cooperative Dialysis Study. Kidney Int Suppl S19–S25 (1983).
Acknowledgements
We would like to thank the members of the Statistic Center at Chang Gung Memorial Hospital and Hemodialysis Center at Chang Gung Memorial Hospital for their invaluable and dedicated assistance. Wen-Hung Huang and Tzung-Hai Yen received funding through research grants from the Chang Gung Memorial Hospital, Linkou (CMRPG3D0322, G3D0012), and Cheng-Hao Weng received funding through research grants from the Chang Gung Memorial Hospital, Linkou (CMRPG5D0081).
Author information
Authors and Affiliations
Contributions
Conceived and designed the experiments: W.-H.H., T.-H.Y., J.-H.L. Performed the experiments: W.-H.H., T.-H.Y. and C.-W.H. Analyzed the data: C.-H.W., T.-H.Y. and C.-H.W. Statistic Center in Chang Gung Memorial Hospital. Contributed reagents/materials/analysis tools: T.-H.Y., J.-H.L. and C.-H.W. Wrote the paper: W.-H.H., J.-H.L. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Huang, WH., Lin, JH., Weng, CH. et al. Environmental NO2 and CO Exposure: Ignored Factors Associated with Uremic Pruritus in Patients Undergoing Hemodialysis. Sci Rep 6, 31168 (2016). https://doi.org/10.1038/srep31168
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep31168
This article is cited by
-
Chronic kidney disease–associated pruritus: what is known and its application in children
Pediatric Nephrology (2024)
-
Emerging role of air pollution in chronic kidney disease
Environmental Science and Pollution Research (2021)
-
Association between exposure to ambient air pollution and renal function in Korean adults
Annals of Occupational and Environmental Medicine (2018)
-
Association Between Serum Aluminum Level and Uremic Pruritus in Hemodialysis Patients
Scientific Reports (2018)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.