Abstract
Here, we present a fast mix-and-measure immunoassay for the specific semiquantitative detection of His-tagged proteins, for example in E. coli cell lysate. The assay is based on Förster resonance energy transfer (FRET) between a lanthanide dye-labeled low-affinity His-peptide and an acceptor-labeled anti-His-tag antibody. The targeted His-tag protein in the sample displaces the donor-labeled peptide and leads to a concentration-dependent time-resolved fluorescence signal. The assay has a total assay time of less than two minutes including sample preparation. The assay recognizes both, N- and C-terminally tagged proteins. The detection limit is comparable to those obtained in SDS-PAGE or Western Blot, which are used as standard methods for the characterization of His-tag protein expression. Additionally, we demonstrate a full compatibility of the developed assay to cell lysate and a correlation to detectable bands in a western blot application. In conclusion, this fast, sensitive, specific and affordable mix-and-measure assay provides a timesaving and user-friendly way to quantify recombinant protein expression. It substantially reduces the workload for recombinant protein detection, especially when His-tag-protein-containing fractions in manual chromatographic purifications have to be identified.
Similar content being viewed by others
Introduction
Cloning and the subsequent recombinant expression of proteins is state-of-the-art in molecular biology and commonly used for decades. As detection and purification of these proteins is often a difficult process, epitope tags are highly versatile and regularly used tools for that purpose1. Here, additional amino acid sequences are usually added to the terminal ends of the desired protein. These amino acid sequences can then represent epitopes for specific binding partners like antibodies. The short hexa-His tag is one of the most commonly used protein tags and allows an easy and fast purification that is based on the strong affinity of histidine sequences to a nickel-complex (Ni-NTA). The observed KD values for this interaction are in the micromolar range and allow a highly specific purification of His-tagged proteins via metal-affinity chromatography2,3. After recombinant expression, the purification of His-tagged proteins using metal affinity chromatography is performed manually in most cases and the identification of the target protein-containing fractions is a tedious process. A simple measurement of the UV-absorption is not specific enough to identify the target protein-containing fractions and can also be rather insensitive in case of proteins with a low tryptophane content and therefore a low extinction coefficient at 280 nm. Another standard technique to characterize and identify target protein containing fractions during and after purification is SDS-PAGE. In some cases, additional Western Blot experiments may be performed, for which a variety of anti-His-tag antibodies exists4,5,6,7. However, the characterization of all protein fractions via SDS-PAGE and, if needed Western blot, although being used as a standard procedure, is a time-consuming procedure, especially if it is only to identify the fractions containing the target protein. A fast mix-and-measure assay for specific detection of His-tagged proteins could therefore simplify the identification process of His-tag containing fractions dramatically. Here, we established a technique for an immediate detection of His6-labeled proteins in crude biological samples (e.g., cell lysate for protein expression specimens) based on a 90 s immunoassay principle8,9 and an in-house developed monoclonal anti-His-tag-antibody.
Results
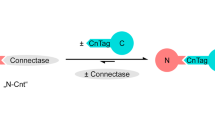
A fast homogeneous immunoassay based on Förster resonance energy transfer was developed for the detection of His-tagged proteins (Fig. 1) to support the characterization of recombinant protein expression and subsequent purification steps. Especially in case of non-automated, manual fraction collection in chromatography, the workload to identify the His-tag containing fractions via SDS-PAGE and Western blot is significant. In this assay, the protein sample is mixed with a small His-peptide that is labeled with the phosphorescent donor dye EuLH10 - a phenanthroline-based europium chelate - and then briefly incubated with the anti-His-tag antibody “8-4-4”, which was labeled with Black hole quencher 10 (BHQ-10) as an acceptor dye for EuLH. The target protein competes with the EuLH donor peptide for the paratope binding site of the antibody, resulting in a target-concentration-dependent phosphorescence signal (also referred to as time-resolved fluorescence, TRF). According to our earlier study on low-affinity donor peptides in homogeneous FRET assays8, a first step required a screening for a suitable donor peptide sequence with a lower affinity to the anti-His-tag antibody compared to the native His6 sequence that is present in His-tagged proteins. The design of such a low-affinity peptide allows for a more complete and easier displacement of the low-affinity peptide by the target analyte and therefore improves the assay performance. In detail, we investigated the FRET efficiency of three different fluorescein-labeled poly-His peptides compared to the native His6 sequence after incubation with increasing amounts of BHQ-10 labeled antibody (Fig. 2). For the native His6 peptide (HHHHHH-NH2), the FRET efficiency increased up to 81%, which indicates a strong and specific interaction between the peptide and the antibody. Reducing the sequence to 5 histidines (HHHHH-NH2) did not influence the FRET efficiency curve substantially and therefore also not the affinity (FRET efficiency = 77%). When we introduced an opposite charge into the peptide sequence (glutamic acid substitute on position 3 (HHEHH-NH2)) we observed a complete loss in FRET efficiency, indicating that this substitution leads to a full loss of affinity to the anti-His-tag antibody. A His3Arg substitution (HHRHH-NH2) showed a reduced, but still significant (p < 0.01, two-sided, unpaired t-test) FRET-efficiency of up to 44% and was then used as a low-affinity peptide for the desired homogeneous FRET assay.
Principle of the homogeneous FRET immunoassay based on a low-affinity donor peptide with phosphorescence detection.
The donor-labeled peptide binds to the acceptor-labeled antibody. The donor emission is reduced due to FRET. The His-tag-containing analyte in the sample displaces the donor peptide, which leads to a concentration-dependent signal increase. EuLH was used as donor dye for phosphorescence detection, BHQ-10 served as an acceptor dye on the antibody.
FRET efficiency depending on BHQ-10-mAb concentration for several donor peptides.
Each donor peptide (10 μL, 40 nmol/L) was mixed with increasing concentrations of BHQ-10-labeled anti-His-tag mAb “8-4-4” (40 μL, 0…160 nmol/L). Fluorescence intensity was measured after a 90 s shaking step. Error bars = SD, n = 3.
We tested two different His6-tagged proteins in our assay, TEV protease (C-terminal His-tag) and trigger factor (N-terminal His-tag) as well as chemically His6-labeled bovine serum albumin ((His6)chem-BSA). For all, increasing phosphorescence intensities with increasing protein concentration were obtained (Fig. 3). In the case of the two recombinant proteins, TEV-His6 and His6-trigger factor, the detection limit was identical at 0.8 μmol/L (TEV-His6 21 mg/L, His6-trigger factor 39 mg/L, Fig. 3a and b). Additionally, a third recombinant protein with C-terminal His-tag, NPP3-His6 was tested in the assay and showed a similar assay response curve (see Supplementary Figure S2). For the model protein (His6)chem-BSA, which was chemically labeled with an excess of nine His6 tags per protein, the detection limit was 32 nmol/L or 2.1 mg/L (Fig. 3c). The sensitivity of this assay is sufficient for the mix-and-measure assay to determine recombinant His-tagged proteins, e.g., in chromatographic purification fractions. In addition, the mix-and-measure assay showed no response for untagged BSA (Fig. 3c). The specificity of the anti-His-tag antibody was further confirmed by ELISA analysis of three additional chemically His6-labeled proteins in direct comparison to their unlabeled counterparts (see Supplementary Figure S1). Taken together, these findings demonstrate a high specificity of the antibody for His6-tagged proteins. The KD value was estimated to be in the range of 1−3×10−8 mol/L. All His6–tagged proteins (TEV protease, trigger factor and NPP3, as well as the chemically His6-labeled BSA) could be detected highly significantly and semi-quantitatively within a total assay time of less than two minutes, independently from the position of the His-tag. Next, we compared the signals obtained after 90 s for the model protein (His6)chem-BSA with those obtained after an additional incubation time of 150 min. No substantial differences were observed, which indicates that signal stability is reached after 90 s (see Supplementary Figure S3). For the examination of the assay performance with crude biological samples, we tested the assay's compatibility to E. coli cell lysate as the most common sample matrix in recombinant protein expression. We recorded signal curves in presence of the target TEV-His6 dissolved in buffer or E. coli cell lysate (Fig. 4). Both curves showed a concentration-dependent signal increase and the E. coli cell lysate did neither affect the limit of detection (LOD) nor the dynamic range. Furthermore, significant background fluorescence from matrix components was not observed due to the time-gated detection of EuLH phosphorescence. Taken together, these findings demonstrate the potential of the assay for semiquantitative detection and a good assay compatibility in E. coli cell lysate samples. Samples containing high imidazole concentrations may require slight sample dilutions to ensure full assay compatibility. Nitrogen-containing aromatic compounds such as imidazole are known to enhance europium signals11 including, in this assay, the corresponding background signals.
Homogeneous His6 detection assay – Response curves for C-terminally His6-tagged TEV protease (a), N-terminally His6-tagged trigger factor (b) and chemically labeled (His6)chem-BSA/tag-free BSA as a control (c). Protein samples (50 μL) were premixed with donor peptide (10 μL, 40 nmol/L) and then mixed with BHQ-10-labeled anti-His-tag mAb “8-4-4” (40 μL, 80 nmol/L). Phosphorescence intensity was measured after a 90 s shaking step. Error bars = SD, n = 3. The grey dashed lines indicate the detection limit (Background signal c = 0 plus 3x SD).
Homogeneous His6 detection assay – Sample compatibility to E. coli cell lysate.
His6 samples (TEV-His6, 50 μL, dissolved in E. coli cell lysate or buffer) were premixed with donor peptide (10 μL, 40 nmol/L) and then mixed with BHQ-10-labeled anti-His-tag mAb “8-4-4” (40 μL, 80 nmol/L). Phosphorescence intensity was measured after a 90 s shaking step. Error bars = SD, n = 3.
The detection limit in the nano- to micromolar range compares well with those observed in SDS-PAGE (Coomassie stain) and Western Blot12. Finally, the results obtained from this fast mix-and-measure assay should correlate with detectable protein bands in a Western Blot experiment. We performed a Western Blot using the same primary monoclonal “8-4-4” anti-His-tag antibody like in the mix-and-measure assay. Detection was examined using a fluorophore-labeled anti-mouse-IgG secondary antibody. Both proteins that were previously detected with the mix-and-measure assay (BSA and TEV protease) could also be detected in the Western Blot (Fig. 5). For His6-TEV, we also analyzed a spiked E. coli cell lysate sample to investigate the specificity of the “8-4-4” anti-His-tag mAb for His-tags within a complex matrix protein mixture (lane 3). Besides the TEV Protease band, no matrix proteins from E. coli were detected non-specifically. This result indicates that the fast homogeneous assay is suitable as a pre-test prior to SDS-PAGE and Western Blot in the characterization of protein expression profiles. Only samples with a significant signal in the fast FRET assay need to be analyzed in SDS-PAGE and/or Western Blot if necessary. This enables an economical handling of multiple samples derived from the expression and purification process. For example, fractions in which the target protein content is below the detection limit of this fast mix-and-measure assay are also not interesting for a further analysis by SDS-PAGE because the protein content is too low to be of interest for recombinant protein expression procedures. This allows either a restriction of SDS-PAGE analysis to certain key fractions in the protein purification process or could potentially also allow to completely replace the tedious SDS-PAGE analysis with this fast assay.
Western Blot analysis using anti-His-tag mAb “8-4-4” as primary antibody.
Standard: Precision Plus Protein™ Dual Xtra (Bio-Rad Laboratories); Lane 1: (His6)chem-BSA (1 μg); lane 2: TEV-His6 in buffer (1 μg); lane 3: TEV-His6 (1 μg) in E. coli cell lysate. RPE-goat-anti-mouse-IgG was used as secondary antibody for fluorescence detection (605/50 nm, recording time 5.33 s).
Discussion
In this work, we developed a fast mix-and-measure assay to determine His-tagged proteins semiquantitatively in E. coli cell lysate in less than two minutes independently from the position of the His-tag in the protein. As previously described, this method simplifies the characterization of protein expression after purification in molecular biology, or even potentially replaces the laborious SDS-PAGE analysis in recombinant protein expression. The fast response of this assay is one of the major advantages compared to alternative sensors for the detection of His-tagged proteins. Alternative sensors rely either on anti-His-tag antibodies for specific detection or antibody-free approaches, e.g., facilitating the specific interaction between His-tags and a Nickel complex (Ni-NTA). One of the fastest sensors described so far, is a label free His-tag sensor chip based on the interaction with Ni-NTA and surface-plasmon resonance with a total assay time of approx. 12 min3. A similar approach to this FRET-based homogenous assay are the sensitive HTRF® assays from Cisbio, France, using an europium cryptate as TRF donor coupled to an anti-His-tag antibody and the phycobiliprotein XL665 coupled to a His6 peptide. Unfortunately, there is no scientific literature available with detailed information for that assay. However, the product description13 suggests a similar sensitivity, but the HTRF assay needs a rather long incubation time of 2 h at reduced temperature and is therefore not suitable as a mix-and-measure assay. The detection limit of the developed assay in the micro- to nanomolar range compares well with other His-tag sensors. Darain et al. introduced an approach which uses a label-free impedance-based sensor with a detection limit of 1.5 μmol/L14. In addition, there are also His-tag-assays with sensitivities in the subpicomolar range as described by Wasowicz et al. using gold nanorods and surface plasmon resonance or impedance spectroscopy as detection system15,16. Another example is an antibody-free approach using a chemiresistive sensor17. While these detection systems are more sensitive than the assay described here, the assay times are in the range of 10–30 min. This is indeed faster than SDS-PAGE or ELISA techniques, but in our opinion still not fast enough to be able to screen protein fractions during the process of purification. Furthermore, a detection limit in the lower mg/L-range as obtained for this assay compares well with typical concentrations of expressed proteins in lysed cell medium14. Additionally, all alternative methods are based on non-traditional equipment for a molecular biology laboratory, whereas fluorescence readers are relatively widely available in these laboratory environments. The unique properties of the used lanthanide dye EuLH, i.e. that it can be excited in the near-Vis region at 360 nm, enables the use of simple and cheap glass optics instead of quartz glass for the measurement10. The use of a His10- instead of a His6- tag could potentially improve the detection limit of the assay described here due to a better sterical accessibility of the epitope.
As in case of SDS-PAGE analysis, the drawback of this mix-and-measure assay is its limitation to semiquantitative analysis. However, this limitation still allows applications as described above like, e.g., purification fraction monitoring. A well known issue that is described in the literature18 is the fact that His-tag antibodies can show different recognition behavior depending on the protein target and the chosen detection method. This does not seem to be the case for the in-house generated and produced monoclonal anti-His-tag antibody “8-4-4” as it showed a comparable response in the developed homogeneous assay, conventional Western blot as well as standard indirect ELISA. Taken together, this simple and fast His-tag assay allows a specific monitoring in E. coli cell lysate sample in almost real time and has the potential to reduce the workload in recombinant protein expression considerably. Therefore, our developed homogeneous His-tag assay represents a major contribution to the methodologies used in protein expression and purification.
Methods
Materials
Phosphorylase B (rabbit muscle), hydrochloric acid (conc.), were purchased from Sigma-Aldrich (Taufkirchen, Germany). Black Hole Quencher 10 (BHQ-10; purity > 75%) succinimidyl ester was ordered from BioCat (Heidelberg, Germany). All Fmoc amino acid derivatives used for solid phase peptide synthesis were supplied by Orpegen Pharma (Heidelberg, Germany). N,N’-Diisopropylcarbodiimide (DIC) and casein (≥90% beta-casein) were obtained from Fluka (Taufkirchen, Germany). Polystyrene-based Fmoc Rink-amide (MBHA) resin was purchased from MultiSynTech (Witten, Germany). SDS (≥99%) was purchased from Carl Roth (Karlsruhe, Germany). Bovine serum albumin (BSA), acrylamide/bis (30% T, 2.67% C), TEMED, ammonium persulfate, glycine and Tween® 20 (pure) were supplied by Serva Electrophoresis (Heidelberg, Germany). Sub-TMB substrate for HRP-ELISA was obtained from Viro-Immun Labor-Diagnostika (Oberursel, Germany). HRP-goat-anti-mouse-IgG secondary antibody for ELISA was purchased from Dianova (Hamburg, Germany). SMPH linker (Succinimidyl–6– [(β–maleimidopropionamido)hexanoate]) was supplied by Thermo Fisher Scientific (Dreieich, Germany). Nonfat dried milk powder and Tris base were purchased from Applichem (Darmstadt, Germany). For western blot, low-fluorescence PVDF blot membrane, AdvanBlockTM-PF protein-free blocking solution, AdvanWashTM immunoblot washing solution and RPE-goat-anti-mouse-IgG antibody were obtained from Biozym Scientific (Hess. Oldendorf, Germany). Deionized water was obtained from a Purelab Ultra water purification system (18.2 MΩ*cm). 384-well non-binding flat-bottom microtiter plates were purchased from Greiner Bio-One (Frickenhausen, Germany). Precision Plus Protein™ Dual Xtra protein standard for Western Blot was supplied from Bio-Rad Laboratories (Munich, Germany). Dulbecco's Modified Eagle Medium (DMEM), fetal bovine serum (South America Origin), non-essential amino acids and penicillin-streptomycin was obtained from Life Technologies (Karlsruhe, Germany). Escherichia coli cell lysate was produced by ultrasonication of BL21AI cells (DSMZ, Braunschweig, Germany) in aqueous phosphate buffer (PBS, 10 mmol/L with 150 mmol/L NaCl, pH 7.4) using a Vibracell 75041 sonicator (30 s pulse time and 40% amplitude, Fisher Scientific, Illkirch, France). The protein concentration of the cell lysate was determined to be 100 mg/L and its protease activity was confirmed in an earlier study19. TEV-His6, His6-trigger factor and NPP3-His6 were provided by Dr. Michael Zahn, Stefanie Weinert and Christoph Döhler, respectively (Research group Prof. Norbert Sträter, Institute for Bioanalytical Chemistry, Leipzig University).
Peptide Synthesis
The peptides used in the homogeneous assay and for His6 protein labeling were synthesized as C-amides on MBHA resin using standard solid-phase synthesis. EuLH10 and carboxyfluorescein as donor in the homogeneous assay were incorporated to the free N-terminus via a free carboxyl group using the same reaction conditions as for each amino acid coupling before. All peptides were cleaved from the synthesis resin with TFA and finally purified via RP-HPLC and characterized using MALDI-MS.
Protein Labeling
For labeling of proteins (BSA, phosphorylase B and casein) with His6, the protein was firstly maleoylated with SMPH and secondly labeled with an excess of 9 eq His6-peptide with an N-terminal cysteine side chain (CHHHHHH-NH2). Unreacted linker and peptide were removed by dialysis (MWCO 10,000, Sigma-Aldrich, Taufkirchen, Germany). Protein concentration was determined using absorption at 280 nm. For the homogeneous assay, the “8-4-4” antibody was labeled with BHQ-10 succinimidyl ester. Unreacted dye was removed via SEC on a HiTrap desalting column (5 mL, GE Healthcare, Munich, Germany).
Antibody generation
The monoclonal mouse anti-His-tag antibody “8-4-4” was generated in collaboration with Biogenes (Berlin, Germany). Murine hybridoma cells (SP2/0) producing κ-IgG1 were cultured in Dulbecco's Modified Eagle Medium (DMEM) containing 15% fetal bovine serum, 1% non-essential amino acids, 1% penicillin-streptomycin and 50 μmol/L beta-mercaptoethanol. The antibody from cell culture supernatant was purified by affinity chromatography with protein G-sepharose (GE Healthcare).
Homogeneous assay
For the homogeneous assay, analyte solutions (50 μL, dissolved in assay buffer: PBS pH 7.4 with 2 mg/mL BSA, or E. coli cell lysate) were premixed with donor peptide (EuLH-HHRHH-NH2, 10 μL, 40 nmol/L in assay buffer), then BHQ-10-labeled “8-4-4” anti-His-tag antibody was added (40 μL, 80 nmol/L in assay buffer). Phosphorescence intensity was recorded using the TRF-EuSa-Cartridge (λEx = 370 nm, 80 nm bandwidth, λEm = 616 nm, 10 nm bandwidth) in a SpectraMax Paradigm plate reader (Molecular Devices) after a 90 s shaking step. The assay was performed in white, non-binding 384-well plates with flat bottom (Greiner Bio-One). All experiments were carried out as triplicates. The detection limit (LOD) was determined to be the lowest tested concentration with a mean signal higher than the background control with c = 0 plus 3x its standard deviation.
Western Blot
For western blot, a standard SDS-PAGE gel was prepared according to the Laemmli protocol20 with modifications according to Ladner et al.21. Precision Plus Protein™ Dual Xtra (Bio-Rad Laboratories) was used as molecular weight standard in 1:10 dilution. The proteins in the gel were transferred to a low-fluorescence PVDF blotting membrane in a semi-dry blot procedure (15 min, 25 V. 1.3 A, Trans-Blot® TurboTM instrument, Bio-Rad Laboratories) using the Trans-Blot® TurboTM RTA transfer kit. The membrane was blocked with AdvanBlock™-PF blocking solution for 30 min, incubated with “8-4-4” anti-His-tag antibody (3.3 mg/L in blocking solution, 60 min) and washed with AdvanWashTM washing solution (3 × 5 min). Then, the membrane was incubated with secondary RPE-goat-anti-mouse-IgG (1:4000 in washing buffer with 0.5% milk powder, 60 min) and washed with washing solution (2 × 5 min) and finally with Tris buffer (20 mmol/L Tris, 500 mmol/L NaCl, pH 7.5, 1 × 5 min). Fluorescence detection was performed using the Chemi-Doc MP instrument (Bio-Rad Laboratories) by measuring the Cy3 channel (605/50 nm, recording time 5.33 s).
Availability of Materials
The monoclonal “8-4-4” anti-His-tag antibody will be publicly available on request for further use and characterizations.
References
Terpe, K. Overview of tag protein fusions: from molecular and biochemical fundamentals to commercial systems. Appl Microbiol Biotechnol 60, 523–533 (2003).
Lichty, J. J., Malecki, J. L., Agnew, H. D., Michelson-Horowitz, D. J. & Tan, S. Comparison of affinity tags for protein purification. Protein Expr Purif 41, 98–105.
Nieba, L. et al. BIACORE Analysis of Histidine-Tagged Proteins Using a Chelating NTA Sensor Chip. Anal Biochem 252, 217–228.
Strandmann, E. P. V. et al. A highly specific and sensitive monoclonal antibody detecting histidine-tagged recombinant proteins. Protein Eng 8, 733–735 (1995).
Zentgraf, H. et al. Detection of histidine-tagged fusion proteins by using a high-specific mouse monoclonal anti-histidine tag antibody. Nucleic Acids Res 23, 3347–3348 (1995).
Lindner, P. et al. Specific detection of his-tagged proteins with recombinant anti-His tag scFv-phosphatase or scFv-phage fusions. Biotechniques 22, 140–149 (1997).
Müller, K. M., Arndt, K. M., Bauer, K. & Plückthun, A. Tandem immobilized metal-ion affinity chromatography/immunoaffinity purification of His-tagged proteins--evaluation of two anti-His-tag monoclonal antibodies. Anal Biochem 259, 54–61 (1998).
Kreisig, T., Hoffmann, R. & Zuchner, T. Homogeneous Fluorescence-Based Immunoassay Detects Antigens Within 90 Seconds. Anal Chem 83, 4281–4287 (2011).
Kreisig, T., Hoffmann, R. & Zuchner, T. Highly Efficient Förster Resonance Energy Transfer in a Fast, Serum-Compatible Immunoassay. ChemBioChem 14, 699–702 (2013).
Zuchner, T. et al. Highly Sensitive Protein Detection Based on Lanthanide Chelates with Antenna Ligands Providing a Linear Range of Five Orders of Magnitude. Anal Chem 81, 9449–9453 (2009).
Lehn, J. M. & De Vains, J.-B. R. Synthesis and Properties of Macrobicyclic Cryptates Incorporating Five- and Six-Membered Biheteroaryl Units. Helv Chim Acta 75, 1221–1236 (1992).
Weiss, W., Weiland, F. & Görg, A. in Methods in Molecular Biology (Reinders J., & Sickmann A., eds. ) 564, 59–82–82 (Humana Press, 2009).
Product information 6HIS-tag check kit, Cisbio, 62HISPEB rev04 (July 2008). Date of access: 13/02/2014, http://www.htrf.com/sites/default/files/ressources/cisbio-pi-62HISPEB.pdf.
Darain, F., Ban, C. & Shim, Y.-B. Development of a new and simple method for the detection of histidine-tagged proteins. Biosens Bioelectron 20, 857–863 (2004).
Wąsowicz, M. et al. Comparison of electrochemical immunosensors based on gold nano materials and immunoblot techniques for detection of histidine-tagged proteins in culture medium. Biosens Bioelectron 24, 284–289 (2008).
Wąsowicz, M., Milner, M., Radecka, D., Grzelak, K. & Radecka, H. Immunosensor incorporating anti-His (C-term) IgG F(ab') fragments attached to gold nanorods for detection of His-tagged proteins in culture medium. Sensors 10, 5409–5424 (2010).
Aravinda, C. L., Cosnier, S., Chen, W., Myung, N. V. & Mulchandani, A. Label-free detection of cupric ions and histidine-tagged proteins using single poly(pyrrole)-NTA chelator conducting polymer nanotube chemiresistive sensor. Biosens Bioelectron 24, 1451–1455 (2009).
Debeljak, N., Feldman, L., Davis, K. L., Komel, R. & Sytkowski, A. J. Variability in the immunodetection of His-tagged recombinant proteins. Anal Biochem 359, 216–223 (2006).
Knappe, D. et al. Rational Design of Oncocin Derivatives with Superior Protease Stabilities and Antibacterial Activities Based on the High-Resolution Structure of the Oncocin-DnaK Complex. ChemBioChem 12, 874–876 (2011).
Laemmli, U. K. Cleavage of Structural Proteins during the Assembly of the Head of Bacteriophage T4. Nature 227, 680–685 (1970).
Ladner, C. L., Yang, J., Turner, R. J. & Edwards, R. A. Visible fluorescent detection of proteins in polyacrylamide gels without staining. Anal Biochem 326, 13–20 (2004).
Acknowledgements
The authors thank Kristin Dobslaff for help with preparation of the “8-4-4” antibody, Stefanie Langanke for help with peptide synthesis, Dr. Constance Nürnberger for synthesis of EuLH as well as Dr. Michael Zahn, Stefanie Weinert and Christoph Döhler for providing TEV-His6, His6-trigger factor and NPP3-His6, respectively. We gratefully thank Prof. Dr. Ralf Hoffmann for lab access and helpful discussions. This project was funded by the GO-Bio initiative from the Federal Ministry of Education and Research (BMBF, Germany, GO-Bio project no. 0315988 to T.Z.).
Author information
Authors and Affiliations
Contributions
T.K. and T.Z. wrote and edited the manuscript. T.K. designed and performed the experiments on the homogeneous assay, analyzed the data and prepared the figures for the manuscript. A.P. designed and performed the experiments on indirect ELISA and analyzed its data. D.V. designed and performed the experiments on Western Blot. K.Z. designed and performed the generation, production and purification of the anti-His-tag antibody. T.Z. conceived the study and supervised all experiments. All the authors read and reviewed the manuscript, agreed that it was ready for publication and accepted responsibility for its contents.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Electronic supplementary material
Supplementary Information
His-tag protein monitoring by a fast mix-and-measure immunoassay.Here, additional data on the specificity of the anti-His-tag-antibody in indirect ELISA, measurement of NPP3-His6 with the developed homogeneous assay as well as data on signal stability of the homogeneous assay over more than 90 seconds is shown.
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License. The images or other third party material in this article are included in the article's Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder in order to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/4.0/
About this article
Cite this article
Kreisig, T., Prasse, A., Zscharnack, K. et al. His-tag protein monitoring by a fast mix-and-measure immunoassay. Sci Rep 4, 5613 (2014). https://doi.org/10.1038/srep05613
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep05613
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.