Abstract
Wastewater from the sweet potato starch industry is a large source of nutrient-rich substrates. We assessed whether this wastewater could be used to produce Paenibacillus polymyxa biofertilizer for foliar application to tea trees. Using the central composite design methods we experientially determined that the optimal culture conditions for P. polymyxa were pH, 6.5; temperature, 29.0°C; and incubation time, 16 h. Under these conditions, a maximum biomass of 9.7 × 109 cfu/mL was achieved. We then conducted a yearlong field investigation to determine the effect of P. polymyxa biofertilizer on the growth of tea plants (Camellia sinensis). Tea yield, quantity of water extract and tea polyphenol levels were significantly higher after foliar application of the biofertilizer compared to that in the controls by an average of 16.7%, 6.3% and 10.4%, respectively. This approach appears to be technically feasible for organic tea production and is an environmentally friendly way to utilize wastewater.
Similar content being viewed by others
Introduction
Sweet potato (Ipomoea batatas) is one of the world's most important staple food crops and a major source of starch products used in industrial production1. Approximately 6 m3 of sweet potato starch wastewater (SPSW) are produced from pre-processing of 1 ton of sweet potato roots, which is then followed by starch extraction, separation and drying. Generation of such large volumes of SPSW has caused serious environmental problems in many Asian countries2. However, treatment of SPSW, which has a chemical oxygen demand (COD) of up to 25,000 mg/L, is a challenge in many developing countries.
On the other hand, SPSW could be used as an appropriate culture medium for microbial growth because of its high organic content and nontoxicity to cells. Some studies have been published on the production of microbial biomass from starch industry wastewater using various microbes such as Aspergillus oryzae, Trichoderma viride and Rhodotorula glutinis3,4,5. To the best of our knowledge, there have been no reports on the use of SPSW for the production of P. polymyxa biofertilizer and the effects of foliar application of P. polymyxa on the growth of tea plants.
P. polymyxa (formerly Bacillus polymyxa) is a gram-positive, spore-forming bacterium belonging to a diverse group of plant growth-promoting bacteria (PGPB). It has a variety of beneficial effects on plants, such as enhancement of nitrogen fixation, promotion of plant growth, suppression of diseases caused by plant pathogens6,7,8. P. polymyxa strain EBL-06 was isolated from wheat leaves by our laboratory9. Since P. polymyxa can survive in the phyllosphere, an appropriate foliar biofertilizer can be developed, although this involves selection of a suitable substrate that supports the growth of the plant and enhances the quality of leaves. Excessive use of chemical fertilizers has increased agricultural costs, as well as causing a variety of environmental problems and concerns of food safety. For example, excessive fertilization can result in the accumulation of large amounts of nitrate in the soil, which could lead to increased leaching of nitrate into water bodies and contamination of the surface and groundwater10,11. Therefore, utilization of PGPB as biofertilizers has emerged as an alternative for providing plant nutrients to increase plant yield and quality in sustainable agroecosystems8. Foliar application of biofertilizer avoids many of the biotic and abiotic factors and constraints of the soil environment, thereby increasing crop growth and yield. In particular, biofertilizers can meet the requirements of organic tea production, which is cultivated without the use of chemical fertilizers. In addition, the use of biofertilizers could provide an effective means of reducing chemical agricultural inputs such as synthetic pesticide, mineral fertilizers and expensive organic fertilizers, which could lead to lower production costs.
The objectives of this study were to investigate the feasibility of using SPSW as raw material for the production of P. polymyxa biofertilizer and to examine whether foliar application of the biofertilizer could improve tea yield and quality. Optimal cultivation conditions for growing P. polymyxa were determined using the one-factor-at-a-time and central composite design (CCD) methods. We conducted a yearlong field investigation to determine if the application of P. polymyxa biofertilizer by foliar spray could affect the yield and quality (i.e., contents of water extract, polyphenols, caffeine and amino acids) of tea plants (C. sinensis).
Results
Optimization of P. polymyxa cultivation for biofertilizer production
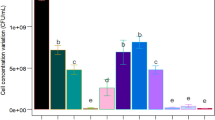
The results of the one-factor-at-a-time experiments showed that the culture conditions for optimal production of P. polymyxa were as follows: incubation time, 16 h; pH, 7.0; temperature, 30°C; and rotational speed, 300 rpm (Fig. S1). In the CCD experiments, the optimal levels of these variables were obtained by analyzing the response surface contour plots using the software Design Expert. This facilitated the identification of the following optimal experimental conditions: pH, 6.5; temperature, 29.0°C; and rotational speed, 250 rpm (Fig. 1). The results of response surface model fitting in the form of ANOVA (analysis of variance) are shown in 12Table 3. Regression analysis demonstrated that the model was significant, as was evident from the calculated F-value of 17.0 and the probability value (P = 0.0001). Therefore, the model should be accurate for predictions within the range of the variables studied. To verify the adequacy of the model equation, three additional experiments were performed in shake flasks under the conditions derived from the model. The mean value of the obtained P. polymyxa biomass was 9.7 × 109 cfu/mL, which was in good agreement with the predicted value (9.8 × 109 cfu/mL). In addition, Fig. S2 shows the growth curves for the P. polymyxa cultivation under optimal conditions.
Effect of P. polymyxa biofertilizer on the growth of tea plants
P. polymyxa biofertilizer was applied to tealeaves to evaluate its effects on growth. Foliar application of the biofertilizer promoted growth in tea plants in all three seasons tested, i.e., Spring tea (SPT), Summer tea (SUT) and Autumn tea (AUT). One-hundred-bud weights of SPT, SUT and AUT were higher than that of the control (CK) by 4.7–10.6%, 7.5–16.5% and 7.1–21.3%, respectively (Fig. 2). We determined the optimal P. polymyxa concentration (4%) for producing weight increase based on the results obtained for SPT and AUT. Three repeated field experiments revealed the treatments by inoculation with the P. polymyxa biofertilizer at a concentration of 4% could increase the tea yield (one-hundred-bud weights) by an average of 10.5% (SPT), 21.1% (AUT) and 16.7% (SUT), respectively (Fig. 2). Compared to that of CK, the one-hundred-bud weight of SUT was significantly higher after foliar application of biofertilizers without sterilization (BO), with an average increase of 16.7%. By comparison, biofertilizers with sterilization (BS) and SPSW for SUT had less effect on plant growth; bud weights increased by 7.5% and 3.4%, respectively. Tea plant growth in response to the dose of P. polymyxa biofertilizer is shown in Table S1.
Effect of P. polymyxa biofertilizer on green tea extracts (GTEs) and tea polyphenols
Levels of GTEs and tea polyphenols in tea plants treated with BS and BO were significantly higher than those in CK (P < 0.05). As shown in Fig. 3 and Fig. 4, the maximum GTE and tea polyphenol levels were obtained with BO, followed by BS, whereas the lowest GTE level was obtained with the SPSW and from the CK. These results were consistent with those of plant growth. Thus, foliar fertilization could be the major factor promoting tea quality. However, our results showed that concentrations of GTEs were significantly increased in SPT, SUT and AUT by 7.4%–11.8%, 2.8%–5.2% and 7.4%–10.6% over control (CK), respectively, while concentrations of tea polyphenols were increased by 8.6%–16.4%, 7.9%–11.3%, 18.6%–28.9% over control (CK), respectively. Overall, the data obtained from both crops showed that application of bacterial strain P. polymyxa as a biofertilizer for growth of C. sinensis could significantly enhance the physiological characters of tea compared to control. Statistical results for levels of GTEs and tea polyphenols with different treatments are shown in Table S1.
Effect of P. polymyxa biofertilizer on caffeine and amino acids content
Increase in concentrations of caffeine and amino acids due to P. polymyxa inoculation on tea in all three seasons was not statistically significant relative to that in the control (Table S1). However, regular fluctuation (increase–decrease) in amino acid content was observed in both control and treated plants throughout the study period. Mean amino acid content (4%–5%) was highest in SPT, followed by SUT and AUT, by 2–3% and 1–3%, respectively (Table S2).
Discussion
SPSW has a relatively high percentage of organics (COD ≈ 19000 mg/L), total nitrogen (TN = 1940 ± 81 mg/L), total phosphorus (TP = 70.6 ± 4.4 mg/L) and other plant nutrients (K = 758 ± 58 mg/L, Fe = 8.6 ± 1.1 mg/L, Mg = 67.3 ± 5.5 mg/L and Zn = 1.8 ± 0.06 mg/L) and represents an important carbon and nutrient-rich resource. Compared to industrial wastewater, the amount of toxic compounds is considerably lower in SPSW (As = 0.23 ± 0.05 mg/L, Cd = 0.24 ± 0.02 mg/L, Cr = 0.25 ± 0.07 mg/L and Pb = 0.91 ± 0.05 mg/L). These properties make SPSW an ideal substrate for the cultivation of P. polymyxa. This observation is supported by a recent report showing the value of starch industry wastewater as a suitable substrate for biological conversion to more valuable products4.
The results demonstrated that the biomass of P. polymyxa (9.7 × 109 cfu/mL) could be easily harvested under simple cultures during a short incubation time (16 h). The metabolites (indole-3-acetic acid [IAA], protease and α-amylase) produced by P. polymyxa not only enhance the productivity of agricultural crops but also improve crop quality by increasing the content of proteins, essential amino acids and vitamins8. Therefore, this approach of bioconversion of SPSW to value-added products can substantially reduce the production cost of biofertilizers, as well as lead to sustainable utilization of residues, which would be beneficial both economically and environmentally.
Foliar biofertilizers of P. polymyxa were investigated for possible beneficial effects on the growth of tea plants. In general, the results were in agreement with those of previously published studies that have investigated the growth response of plants to inoculation with P. polymyxa12,13. The ability of the biofertilizer to promote tea plant growth might have three mechanisms. Firstly, the raw materials (SPSW) for the production of P. polymyxa biofertilizer contains much nutrient elements such as N, P, K, Fe, Mg, Zn (Table 1), which are readily absorbed by tea plants. It has been demonstrated that plant leaves are able to take up inorganic nutrients provided in aqueous forms, which are then transported to shoots and roots14. Based on our results, nitrogen (1900 mg/L) and phosphorus (70 mg/L) in soluble forms from the biofertilizers are readily absorbed by tea plants, thus increasing the tea growth of about 3.4%. Secondly, the increase in plant growth may be due to the supply of specific nutrients to the crop, such as the auxin metabolites (IAA) and antibiotic compounds released by P. polymyxa.P. polymyxa strains are known to produce peptide antibiotics and fusaricidins, which strongly inhibit plant pathogens such as Aspergillus niger, Aspergillus flavus, Cladosporium fulvum, Candida albicans and Geotrichum candidum15,16. These components may have been beneficial for stimulation of plant growth in this study which could explain the increase in plant growth by 7.5% (sterilized biofertilizer, BS). Finally, the biological activity of P. polymyxa could increase the yield of about 9.2%, which equal to 16.7% (biofertilizer, BO) - 7.5% (sterilized biofertilizer, BS), owing to P. polymyxa strains could induce systemic resistance in enhancing the disease resistance in tea plants.
The mechanisms of the P. polymyxa strains to promote plant growth and control a variety of pathogens that invade plants were reviewed by Raza et al.17 and Lal et al.18 recently. Recent investigations on the mechanisms of plant growth-promoting rhizobacteria revealed that Pseudomonas and Bacillus strains could induce systemic resistance in enhancing the disease resistance in tea plants against blister disease19. A similar mechanism might take place from the use of P. polymyxa biofertilizer for tea growth in this study. However, the induction of systemic resistance mechanism will be verified in further studies.
GTEs contain a variety of biochemical components, such as tea polyphenols, amino acids and caffeine, all of which are closely associated with tea quality. As the leading functional component of GTEs, as well as because of their beneficial medicinal properties, tea polyphenols have gained substantial interest. In vitro and animal studies have suggested that antioxidants found in polyphenol-containing substances may play an important role in preventing cardiovascular disease, chronic gastritis and some cancers20,21. In this study, foliar application of biofertilizer significantly increased the concentrations of tea polyphenols, suggesting that the nutrients nitrogen and phosphorus in biofertilizers promote the synthesis of tea polyphenols. In addition, Li and Xia22 demonstrated that an adequate amount of nitrogen and phosphorus can provide sufficient raw materials and energy for biosynthesis of tea polyphenols, increasing their content in tealeaves.
Caffeine is a purine alkaloid present in high concentrations in the tea plant23. The caffeine biosynthesis pathway is part of purine metabolism and is catalyzed by three S-adenosyl-l-methionine (SAM)-dependent N-methylation steps: xanthosine → 7-methylxanthosine → 7-methylxanthine → theobromine → caffeine23. Amino acids, which constitute 1–2% of the dry weight of the tealeaf, are synthesized from glutamic acid and ethylamine by theanine synthetase24. The biosynthesis of caffeine and amino acids are mainly accomplished in rhizosphere not in leaf surface which can explain less increase of caffeine and amino acids by the application of foliar biofertilizers.
In summary, production of P. polymyxa biofertilizer from SPSW is technically feasible and the use of this biofertilizer may be beneficial for organic tea production. Furthermore, data from field investigations revealed that foliar application of the biofertilizer can enhance yield and quality of tea production. The technical approach from this study shows the commercial potential for bioconversion of wastewater to environmental friendly, value-added biofertilizers.
Methods
Plant growth-promoting bacterium
P. polymyxa strain EBL-06 isolated from wheat leaves9 was deposited at the China General Microbiological Culture Collection Center (CGMCC) with accession number CGMCC No. 2377. The culture was maintained on potato-dextrose agar (PDA) medium at 4°C and subcultured every 6 months.
Raw material
Starch industry wastewater, contained approximately 20 g/L COD and 2 g/L total nitrogen, was obtained from a local sweet potato starch production company (Xiangfeng Corporation, Hunan, China). The physiochemical characteristics of SPSW are presented in Table 1.
One-factor-at-a-time experiments for biofertilizer preparation
The starter culture and the inoculum were prepared according to our previous study9. P. polymyxa cells from PDA medium were used to inoculate 500 mL Erlenmeyer flasks containing 100 mL of sterilized (121°C, 20 min) SPSW. After 24–32 h incubation at 30°C in a rotary shaker (250 rpm), inoculum from this broth was used as a seed culture. For the optimization of biofertilizer production, relevant factors such as temperature, pH and rotational speed were investigated using the one-factor-at-a-time method. A time course experiment up to 24 h was carried out in Erlenmeyer flasks (500 mL) containing 148 mL of sterilized SPSW with 2 mL P. polymyxa inoculum. To determine the optimal starting pH for biofertilizer production, the pH (5–8) of the medium was adjusted by the addition of 1 M HCl and 1 M NaOH before sterilization. To determine the optimal temperature for biofertilizer production, liquid cultures were incubated at 20°C, 24°C, 28°C, 32°C, 36°C and 40°C. For optimization of rotational speed, cultures were shaken at 50, 100, 150, 200, 250 and 300 rpm. All experiments were conducted in duplicate and key results were repeated three times to establish their validity.
CCD for optimization of biofertilizer production
After determining the preliminary range of culture variables according to one-factor-at-a-time experiments, a CCD was performed with three operation parameters (X1, temperature; X2, initial pH; X3, rotational speed) at five levels (–1.68, –1, 0, +1, +1.68) and 19 experiments (Table 2). Five replicates at the center point were conducted for calculating the purely experimental uncertainty variance. Data from CCD were analyzed with the following second-degree polynomial equation:

where Y represents the predicted response; β0 is an intercept; β1, β2 and β3 are linear coefficients; β12, β13 and β23 are cross-product coefficients; β11, β22 and β33 are quadratic coefficients; and X1, X2 and X3 are input variables16.
Assays of the physiochemical and biochemical properties of the biofertilizer
Changes in pH and total solids were monitored according to standard methods25. The COD was determined using the close reflux and titrimetric method25. Total nitrogen (TN) and total phosphorus (TP) were also quantified according to methods recommended by Xi and Sun25. Concentrations of metals (K, Cd, Cr, Cu, Fe, Mg, As, Pb and Zn) were analyzed using a Prodigy Plasma emission spectrometer (Teledyne Leeman Labs, NH). Total bacteria were enumerated by plate count using PDA culture medium. The concentration of IAA was determined according to the procedure described by Lebuhn et al26. Protease levels were determined according to standard methods27. Physico-chemical characteristics of the SPSW and biofertilizer are presented in Table 1.
Plant materials and treatments
Field investigations were conducted in tea plantations (4 years old) of semi-tropical uplands, Hunan, China (113°19′E, 28°33′N). We selected the tea plant (C. sinensis) to study the efficacy of PGPB strains on tea yield and quality for three seasons (Season: mid-March to mid-October; elevation: 135 m above mean sea level; rainfall: 1386 mm; mean temperature: 16.5–34.5°C; relative humidity: 65–95%). The plantation has red soil that commonly develops on old crystalline rocks under heavy rainfall conditions. There were no obvious differences between tea plants owing to unified management practices.
The field investigations were conducted in a completely randomized design with two factors. The first factor had four levels: without biofertilizers (water, CK), SPSW only, biofertilizers with sterilization (BS) and biofertilizers without sterilization (BO). The second factor comprised the addition of biofertilizers at a rate of 0, 2, 4, or 8%. There were three replicates, each consisting of 50 bushes. The experiment was laid out in a randomized block design with a plot size of 10 m × 20 m. The experimental process was carried out in three periods: Autumn tea (mid-August to mid-October 2010, AUT), Spring tea (mid-March to mid-April 2011, SPT) and Summer tea (mid-June to mid-July 2011, SUT). In autumn and spring periods, various concentrations of BO were applied as foliar spray (30 mL/m2), while sterile water was used as control. In the summer period, we selected the appropriate concentration (obtained from autumn and spring tea treatments) of BS or BO as foliar spray, with sterile water and SPSW serving as control.
Collection and analysis of tea samples
The tea bush (bud with two leaves) was plucked at the study sites on the 1st, 2nd and 3rd week after the application of the biofertilizer. Tea production was measured by one-hundred-bud weight and bud density, however, in this study the tea plants were trimmed uniformly whose bud density had no obvious differences and changes during a short time. So one-hundred-bud weights can be used as indicators of biomass. As well as fresh tealeaves were processed using standard methods, including fixation, rolling and baking to prepare the green tea by the same procedure for each sample28.
Green tea water extracts (GTEs) and determination of nutritional components
Green tea powders (3 g) were extracted three times with 250 mL of boiling water at 100°C for 15 min. The infusions were filtered using a vacuum pump through perfect porosity filter paper at 55°C ± 2°C. The water extract collected were used to assess GTEs, total polyphenols, caffeine and amino acids according to the China National Standard Methord29,30,31,32.
Statistical analyses
Design Expert software version 7.0 (Stat-Ease Inc., Minneapolis, MN, USA) was used for the experimental designs and regression analysis of data. Data of the effect of the treatments on growth and quality of tea were evaluated by ANOVA followed by Duncan's multiple range tests using SPSS 12.0 (SPSS China, Beijing, China). Differences between treatment results were considered significant at P < 0.05.
References
Wang, R., Wang, Y., Ma, G., He, Y. & Zhao, Y. Efficiency of porous burnt-coke carrier on treatment of potato starch wastewater with an anaerobic–aerobic bioreactor. Chem. Eng. J. 148, 35–40 (2009).
Chavalparit, O. & Ongwandee, M. Clean technology for the tapioca starch industry in Thailand. J. Cleaner Prod. 17, 105–110 (2009).
Jin, B., van Leeuwen, H. J., Patel, B. & Yu, Q. Utilisation of starch processing wastewater for production of microbial biomass protein and fungal α-amylase by Aspergillus oryzae. Bioresour. Technol. 66, 201–206 (1998).
Verma, M., Brar, S. K., Tyagi, R. D., Surampalli, R. Y. & Valéro, J. R. Starch industry wastewater as a substrate for antagonist, Trichoderma viride production. Bioresour. Technol. 98, 2154–2162 (2007).
Xue, F. et al. Pilot-scale production of microbial lipid using starch wastewater as raw material. Bioresour. Technol. 101, 6092–6095 (2010).
Jisha, M. & Alagawadi, A. Nutrient uptake and yield of sorghum (Sorghum bicolor L. Moench) inoculated with phosphate solubilizing bacteria and cellulolytic fungus in a cotton stalk amended vertisol. Microbiol. Res. 151, 213–217 (1996).
Lindberg, T. & Granhall, U. Isolation and characterization of dinitrogen-fixing bacteria from the rhizosphere of temperate cereals and forage grasses. Appl. Environ. Microbiol. 48, 683–689 (1984).
Ryu, C.-M., Kim, J., Choi, O., Kim, S. H. & Park, C. S. Improvement of biological control capacity of Paenibacillus polymyxa E681 by seed pelleting on sesame. Biol. Control. 39, 282–289 (2006).
Gu, L. et al. Production of a newly isolated Paenibacillus polymyxa biocontrol agent using monosodium glutamate wastewater and potato wastewater. J. Environ. Sci. 22, 1407–1412 (2010).
Chang, C.-H. & Yang, S.-S. Thermo-tolerant phosphate-solubilizing microbes for multi-functional biofertilizer preparation. Bioresour. Technol. 100, 1648–1658 (2009).
Chowdhury, M. A. H., Kouno, K., Ando, T. & Nagaoka, T. Microbial biomass, S mineralization and S uptake by African millet from soil amended with various composts. Soil Biol. Biochem. 32, 845–852 (2000).
Figueiredo, M. V., Burity, H. A., Martínez, C. R. & Chanway, C. P. Alleviation of drought stress in the common bean (Phaseolus vulgaris L.) by co-inoculation with Paenibacillus polymyxa and Rhizobium tropici. Appl. Soil Ecol. 40, 182–188 (2008).
Khan, Z. et al. Plant growth promoting rhizobacterium, Paenibacillus polymyxa strain GBR-1, suppresses root-knot nematode. Bioresour. Technol. 99, 3016–3023 (2008).
Fernández, V. & Eichert, T. Uptake of hydrophilic solutes through plant leaves: current state of knowledge and perspectives of foliar fertilization. Crit. Rev. Plant Sci. 28, 36–68 (2009).
Deng, Y. et al. Identification of LI-F type antibiotics and di-n-butyl phthalate produced by Paenibacillus polymyxa. J. Microbiol. Methods 85, 175–182 (2011).
Raza, W., Makeen, K., Wang, Y., Xu, Y. & Shen, Q. Optimization, purification, characterization and antioxidant activity of an extracellular polysaccharide produced by Paenibacillus polymyxa SQR-21. Bioresour. Technol. 102, 6095–6103 (2011).
Raza, W., Yang, W. & Shen, Q.-R. Paenibacillus polymyxa: antibiotics, hydrolytic enzymes and hazard assessment. J. Plant Pathol. 93, 419–430 (2008).
Lal, S. & Tabacchioni, S. Ecology and biotechnological potential of Paenibacillus polymyxa: a minireview. Indian J. Microbiol. 49, 2–10 (2009).
Saravanakumar, D., Vijayakumar, C., Kumar, N. & Samiyappan, R. PGPR-induced defense responses in the tea plant against blister blight disease. Crop Prot. 26, 556–565 (2007).
Chen, Q., Zhao, J., Liu, M., Cai, J. & Liu, J. Determination of total polyphenols content in green tea using FT-NIR spectroscopy and different PLS algorithms. J. Pharm. Biomed. Anal. 46, 568–573 (2008).
Khan, N. & Mukhtar, H. Tea polyphenols for health promotion. Life sciences 81, 519–533 (2007).
Li, j. & Xia, J. Summary on Nitrogen (N), Phosphorus (P), Potassium (K) and Tea Quality. Chinese Agricultural Science Bulletin 21, 62 (2005).
Shi, C. et al. Deep sequencing of the Camellia sinensis transcriptome revealed candidate genes for major metabolic pathways of tea-specific compounds. BMC genomics 12, 131 (2011).
Sasaoka, K., Kito, M. & Inagaki, H. Studies on the biosynthesis of theanine in tea seedlings: synthesis of theanine by the homogenate of tea seedlings. Agric. Biol. Chem. 27, 467–468 (1963).
Xi, D. & Sun Y. . Environmental Monitoring, [94–96, 70–71, 113–118, 104–110, 112–113] (Higher Education Press, Beijing, 2010).
Lebuhn, M., Heulin, T. & Hartmann, A. Production of auxin and other indolic and phenolic compounds by Paenibacillus polymyxa strains isolated from different proximity to plant roots. FEMS Microbiol. Ecol. 22, 325–334 (1997).
General Administration of Quality Supervision, Inspection and Quarantine of P. R. China. & Standardization Administration of P. R. China. Microbial inoculants in agriculture. (China Standards Press, Beijing, 2006).
General Administration of Quality Supervision, Inspection and Quarantine of P. R. China. & Standardization Administration of P. R. China. . Production technical practice for tea. (China Standards Press, Beijing, 2011).
Zhou, W. et al. [Tea — Determination of water extracts content] Standards collection for tea. [The First Editing Group of China Standards Press (ed.)] [502–503] (China Standards Press, Beijing, 2011).
Zhou, W. et al. [Determination of total polyphenols and catechins content in tea] Standards collection for tea. [The First Editing Group of China Standards Press (ed.)] [533–539] (China Standards Press, Beijing, 2011).
Zhou, W. et al. [Tea — Determination of caffeine content] Standards collection for tea. [The First Editing Group of China Standards Press (ed.)] [529–531] (China Standards Press, 2011).
Zhou, W. et al. [Tea — Determination of free amino acids content] Standards collection for tea. [The First Editing Group of China Standards Press (ed.)] [541–542] (China Standards Press, Beijing, 2011).
Acknowledgements
This work was supported by the Knowledge Innovation Program of the Chinese Academy of Sciences (Nos. KZZD-EW-09-3 and KSCX2-EW-B-1-5) and the Key Technologies R&D Program of China (No. 2012BAC25B01). We would like to thank Mr. Weidong Zhang, Dingyi Yu, Yu Zhang and Xinghui Li for their technical support.
Author information
Authors and Affiliations
Contributions
S.X. and Z.B. designed and carried out the experiments, analyzed the data and wrote the main manuscript text; B.J. wrote the paper; R.X. and G.Z. designed the experiments. All authors reviewed the manuscript.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Electronic supplementary material
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-ShareALike 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/3.0/
About this article
Cite this article
Xu, S., Bai, Z., Jin, B. et al. Bioconversion of wastewater from sweet potato starch production to Paenibacillus polymyxa biofertilizer for tea plants. Sci Rep 4, 4131 (2014). https://doi.org/10.1038/srep04131
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep04131
This article is cited by
-
Sustainable tea production through agroecological management practices in Vietnam: a review
Environmental Sustainability (2021)
-
The performance and archaeal community shifts in a modified anaerobic baffled reactor treating sweet potato starch wastewater at ambient temperatures
Scientific Reports (2017)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.