« Prev Next »
Introduction
Adaptations for improved vision
All living primates have forward-facing eyes (Figure 1; Johnson, 1901; Cartmill, 1992). In this respect, primates more closely resemble cats and owls than many other mammals (e.g., squirrels or gazelles). Having forward-facing eyes gives primates a wide field of binocular vision (Heesy, 2004, 2009). In other words, most of a primate's visual field is viewed by both eyes simultaneously. By comparison, in species with laterally facing eyes, much of the visual field is monocular (Hughes, 1977). A large binocular visual field in primates is probably an adaptation for enhanced depth perception, which is facilitated by the binocular visual cues of vergence and stereopsis (Walls, 1942; McIlwain, 1996; Tovée, 1996). Although monocular cues (e.g., perspective and motion parallax) may also be used to judge the distance to a visual target, binocular cues are particularly useful for fine depth perception at relatively close ranges (Barlow & Mollon, 1982; McIlwain, 1996).

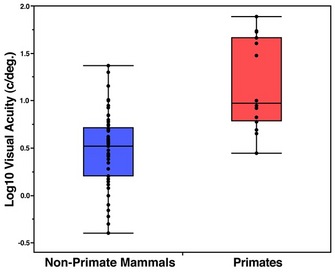
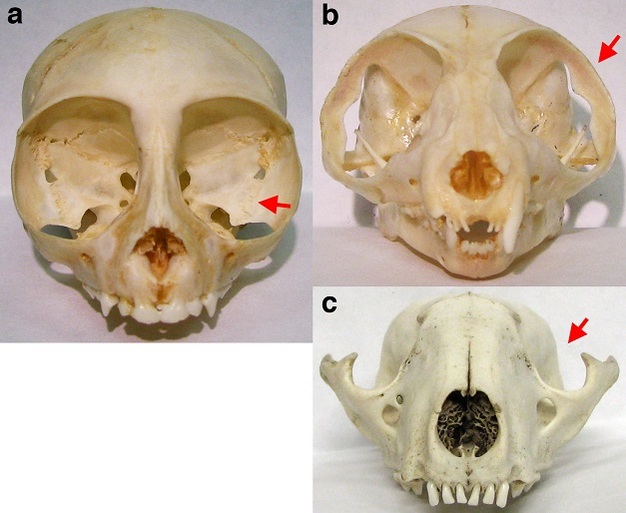
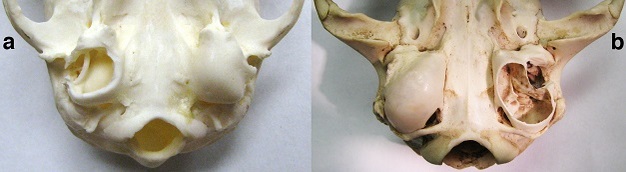
As a group, primates also have high visual acuity (Figure 2). Acute vision in primates is the product of several discrete visual adaptations. First, primates have larger eyes than many other mammals of comparable body size (Ross & Kirk, 2007). Having large eyes ensures that a large image is formed on the retina (Walls, 1942; Land & Nilsson, 2002). This large retinal image may then be sampled by many photoreceptors, improving visual resolution. Second, primates have a dense concentration of photoreceptors in the central retina (Kirk & Kay, 2004). Accordingly, when a primate directs its gaze toward an object of interest, the retinal image of that object is sampled by a large number of small and tightly packed photoreceptors. Third, primates have eyes that are more completely encircled by bone than in most other mammals (Cartmill, 1980, 1992). In strepsirrhines (i.e., lemurs and lorises), this bony enclosure takes the form of a postorbital bar only, while haplorhines (i.e., tarsiers, monkeys, apes, and humans) also possess a postorbital plate (Figure 3). These bony features help stabilize the eyes by insulating them from movements of chewing muscles adjacent to the orbit (Cartmill, 1980; Heesy, 2005; Menegaz & Kirk, 2009).
In addition to having adaptations for enhanced depth perception and visual acuity, primates also have large visual regions of the brain (Allman, 1999; Kaas, 2005, 2008). Macaque monkeys, for example, have at least 32 functionally distinct areas of the cerebral cortex that are devoted primarily to processing visual information (Van Essen et al., 1992; Van Essen, 2004). These visual areas comprise 50% of the total macaque cerebral cortex, whereas only 3% of the macaque cerebral cortex is devoted primarily to auditory functions. Many non-primate mammals have proportionately smaller visual regions of the brain and fewer functionally distinct cortical visual areas (Kaas, 2005, 2008). The comparatively large area of the primate brain devoted to vision is partly explained by the fact that primates must process a larger amount of visual "input" to the brain than many other mammals (Kirk, 2006).
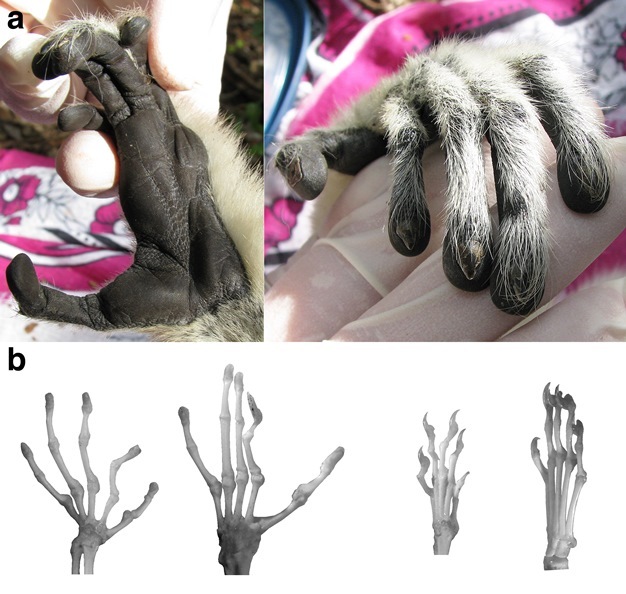
Adaptations for grasping extremities
Adaptations for a slow life history
Misconceptions about Primates
One may also encounter the twin assertions that all primates have a poor sense of smell and that only primates have binocular vision. Although haplorhines do indeed have a reduced olfactory system, strepsirrhines have olfactory anatomy that is not fundamentally different from that of many other mammals (Cave, 1973; Smith et al., 2001, 2007). Similarly, binocular vision appears to be nearly universal among mammals (Hughes, 1977; Heesy, 2004). What differs between species is not the presence of binocular vision, but rather the size of the binocular visual field. Even rabbits, with laterally facing eyes and 360˚ panoramic vision, have a narrow field of binocular vision (Hughes, 1977).
With regard to claims of primate exceptionalism, it should be noted that there is only one anatomical feature that is known to be present in all living primates but is absent in all other living groups that have been studied. In most mammals, the middle ear has a bony floor called an auditory bulla (Figure 5). Primates are unique among living mammals in having an auditory bulla that is completely formed by the petrosal bone (MacPhee, 1981). While auditory bullae have probably evolved multiple times independently in mammals to protect the contents of the middle ear, the specific identity of the bone that forms the bulla has no clear adaptive significance.
Evolutionary considerations
Glossary
adaptations - attributes of an organism that increase the likelihood that it will successfully survive and produce healthy offspring
abducted - displaced away from the midline; an abducted hallux diverges from the other toes and facilitates the ability of the foot to grasp an object, such as a branch
arboreal - living predominantly or exclusively in trees
artiodactyls - hoofed mammals with an even number of toes; e.g., gazelle, deer, cows, pigs
auditory bulla - a bony prominence beneath the cranium of most mammals that forms the floor of the middle ear space
binocular - viewed by both eyes simultaneously
bipedalism - locomotion on two limbs
callitrichine - member of a family of small Neotropical monkeys, including marmosets, pygmy marmosets, tamarins, and lion tamarins
cerebral cortex - the outer covering of the largest part of the brain (cerebrum) in most mammals; commonly referred to as the brain's "grey matter"
crown primates - the group that includes all the descendants (both living and extinct) of the last common ancestor of living primates
depth perception - the ability to judge the distance to an object using visual cues
encephalization - brain size measured relative to body size
exudates - substances, such as gums or resins, that drain from damaged plant tissues
friction ridges - fine ridges occurring on the surface of the hairless palms, soles, and digits of some mammals; these ridges increase adhesion between the skin and an object being grasped
gestation - the period of mammalian development from conception to birth
hallux - the great toe
haplorhines - a group of related primates that includes tarsiers, monkeys, apes, and humans
homoplasy - the independent evolution of similar attributes in separate evolutionary lineages; these features may evolve through similar developmental pathways ("evolutionary parallelism") or different developmental pathways ("evolutionary convergence")
infant dependency - the period of mammalian development from birth to the point at which the offspring may successfully survive independent of its mother
interbirth interval - the average time between successive births
laterally - facing away from the midline of the body
life history - the series of successive changes that an organism undergoes during its development from conception to death
manual - of, or relating to, the hands
mechanoreceptors - cells in the skin related to the sense of touch that respond to mechanical stimuli (e.g., pressure, stretch, vibration)
middle ear - an air-filled space between the ear drum and cochlea (organ of hearing) that includes the three tiny bones (malleus, incus, and stapes) that transmit acoustic vibrations to the inner ear
monocular - viewed by only one eye
motion parallax - a monocular cue for depth perception in which near objects appear to move farther relative to an observer in motion than do far objects
orbit - the bony eye socket
pedal - of or relating to the feet
perspective - a monocular cue for depth perception in which parallel lines appear to converge toward the horizon
petrosal - a bone that surrounds the inner ear in all mammals and also forms the complete auditory bulla in crown primates
photoreceptors - light-sensitive cells (i.e., rods and cones) in the retina
prehensile - referring to the ability to hold and manipulate an object using a single appendage
Primates - a mammalian taxonomic group (order) that includes the living lemurs, lorises, tarsiers, monkeys, apes, and humans
plesiadapiforms - a group of extinct mammals that may be stem primates; all known plesiadapiform species are more than 38 million years old
retinal image - the image projected onto the retina by the eye's cornea and lens
social - living in semi-stable groups characterized by established relationships between individuals that are based on repeated interactions
stem - refers to the extinct members of an evolutionary lineage that branched from the lineage prior to the appearance of the last common ancestor of the living members of that lineage
stereopsis - the perception of depth and three-dimensionality that is created when the brain combines the slightly different images from the left and right eye; one binocular cue for depth perception
strepsirrhines - a group of related primates that includes lemurs and lorises
vergence - the movement of the eyes toward the midline when focusing on near objects; one binocular cue for depth perception
visual acuity - the ability to detect visual details; also called "resolving power"
visual field - the total region of space viewed by an organism's eyes
References and Recommended Reading
Allman, J. M. Evolving Brains. New York: Scientific American Library (1999).
Barlow H. B. & Mollon J. D. (Eds.) The Senses. Cambridge UK: Cambridge University Press (1982).
Barrickman, N. L, Bastian, M. L., Isler, K. & van Schaik, C. P. Life history costs and benefits of encephalization: a comparative test using data from long-term studies of primates in the wild. Journal of Human Evolution. 54, 568-590 (2008).
Byrne, R. W. & Whiten, A. (Eds.) Machiavellian intelligence. Oxford, UK: Oxford University Press (1988).
Calderone, J. B., Reese, B. E. & Jacobs, G. H. Topography of photoreceptors and retinal ganglion cells in the spotted hyena (Crocuta crocuta). Brain, Behavior and Evolution 62, 182-192 (2003).
Cartmill, M. Pads and claws in arboreal locomotion. In Primate Locomotion. Ed. Jenkins, F. A., Jr (New York: Academic Press, 1974). 45-83.
Cartmill, M. Morphology, function and evolution of the anthropoid postorbital septum. In Evolutionary Biology of the New World Monkeys and Continental Drift. Eds. Ciochon, R. L. & Chiarelli, A. B. (New York: Plenum Press, 1980). 243-274.
Cartmill, M. Climbing. In Functional Vertebrate Morphology. Eds. Hildebrand, M., Bramble, D. M., Liem, K. F. & Wake, D. B. (Cambridge MA: Belknap, 1985). 73-88.
Cartmill, M. New views on primate origins. Evolutionary Anthropology 1, 105-111 (1992).
Cave, A. J. E. The primate nasal fossa. Biological Journal of the Linnaean Society 5, 377-387 (1973).
Charnov, E. L. & Berrigan, D. Why do primates have such long lifespans and so few babies? Evolutionary Anthropology 1,191-194 (1993).
Futuyma, D. J. Evolutionary Biology. Sunderland, Massachussetts: Sinauer Associates (1998).
Harvey, P. H. & Clutton-Brock, T. H. Life history variation in primates. Evolution 39, 559-581 (1985).
Heesy, C. P. On the relationship between orbit orientation and binocular visual field overlap in mammals. Anatomical Record 281A, 1104-1110 (2004).
Heesy, C. P. Function of the mammalian postorbital bar. Journal of Morphology 264, 363-380 (2005).
Heesy, C. P. Seeing in stereo: the ecology and evolution of primate binocular vision and stereopsis. Evolutionary Anthropology 18, 21-35 (2009).
Hoffmann, J. N., Montag, A. G. & Dominy, N. J. Meissner corpuscles and somatosensory acuity: the prehensile appendages of primates and elephants. The Anatomical Record Part A 281A, 1138-1147 (2004).
Hughes, A. 1977. The topography of vision in mammals of contrasting lifestyle: comparative optics and retinal organisation. In The Visual System in Vertebrates. Ed. Crescitelli, F. (New York: Springer, 1977) 613-756.
Jerison, H. J. Evolution of the Brain and Intelligence. New York: Academic Press (1973).
Johnson, G. L. Contributions to the comparative anatomy of the mammalian eye, chiefly based on ophthalmoscopic examination. Philosophical Transactions of the Royal Society of London. Series B, Containing Papers of a Biological Character 194, 1-82 (1901).
Jungers, W. L., Lemelin, P., Godfrey, L. R. et al. The hands and feet of Archaeolemur: metrical affinities and their functional significance. Journal of Human Evolution 49, 36-55 (2005).
Kaas, J. H. From mice to men: the evolution of the large, complex human brain. Journal of Biosciences 30, 155-165 (2005).
Kaas, J. H. The evolution of the complex sensory and motor systems of the human brain. Brain Research Bulletin 75, 384-390 (2008).
Kappeler, P. M. & Pereira, M. E. (Eds.) Primate Life Histories and Socioecology. Chicago: The University of Chicago Press (2003).
Kirk, E. C. Visual influences on primate encephalization. Journal of Human Evolution 51, 76-90 (2006).
Kirk, E. C. & Kay, R. F. The evolution of high visual acuity in the Anthropoidea. In Anthropoid Origins: New Visions. Eds. Ross, C. F. & Kay, R. F. (New York: Kluwer Academic / Plenum Publishers, 2004). 539-602.
Kirk, E. C., Lemelin, P., Hamrick, M. W., Boyer, D. M. & Bloch, J. I. Intrinsic hand proportions of primates and other euarchontan mammals: implications for the locomotor behavior of plesiadapiforms. Journal of Human Evolution 55: 278-299 (2008).
Land, M. F. & Nilsson, D.-E. Animal Eyes. New York: Oxford University Press (2002).
Lemelin, P. Morphological correlates of substrate use in didelphid marsupials: implications for primate origins. Journal of the Zoological Society of London 247, 165-175 (1999).
Martin, R. D. Primate Origins and Evolution. Princeton NJ: Princeton University Press (1990).
MacPhee, R. D. E. Auditory regions of primates and eutherian insectivores. Morphology, ontogeny, and character analysis. Contributions to Primatology, Vol. 18. Basel: Karger (1981).
McIlwain, J. T. An Introduction to the Biology of Vision. Cambridge UK: Cambridge University Press (1996).
Menegaz, R. A. & Kirk, E. C. Septa and processes: convergent evolution of the orbit in haplorhine primates and strigiform birds. Journal of Human Evolution 57, 672-687 (2009).
Mumby H. & Vinicius, L. Primate growth in the slow lane: a study of inter-species variation in the growth constant A. Evolutionary Biology 35, 287-295 (2008).
Nowak, R. M. Walker's Mammals of the World. Baltimore MD: The Johns Hopkins University Press (1991).
Passingham, R. E. The Human Primate. Oxford UK: W. H. Freeman and Company (1982).
Pettigrew, J. D. & Manger, P. R. Retinal ganglion cell density of the black rhinoceros (Diceros bicornis): Calculating visual resolution. Visual Neuroscience 25, 215-220 (2008).
Pettigrew, J. D., Bhagwandin, A., Haagensen, M. & Manger, P. R. Visual acuity and heterogeneities of retinal ganglion cell densities and the tapetum lucidum of the African elephant (Loxodonta africana). Brain, Behavior and Evolution 75, 251-261 (2010).
Ross, C. Primate Life Histories. Evolutionary Anthropology 6, 54-63 (1998).
Ross, C. F. & Kirk, E. C. Evolution of eye size and shape in primates. Journal of Human Evolution 52, 294-313 (2007).
Shinozaki, A., Hosaka, Y., Imagawa, T. & Uehara, M. Topography of ganglion cells and photoreceptors in the sheep retina. The Journal of Comparative Neurology 518, 2305-2315 (2010).
Smith, T. D., Siegel, M. I. & Bhatnagar, K. P. A reappraisal of ontogeny, morphology, functionality, and persisting questions on the vomeronasal system of catarrhine primates. Anatomical Record (New Anatomist) 265, 176-192 (2001).
Smith, T. D., Rossie, J. B. & Bhatnagar, K. P. Evolution of the nose and nasal skeleton in primates. Evolutionary Anthropology 16, 132-146 (2007).
Smuts, B. B., Cheney, D. L., Seyfarth, R. M. & Wrangham, R. W. (eds.) Primate Societies. Chicago: The University of Chicago Press (1987).
Soligo, C. & Müller, A. E. Nails and claws in primate evolution. Journal of Human Evolution 36, 97-114 (1999).
Tovée, M. J. An Introduction to the Visual System. Cambridge UK: Cambridge University Press (1996).
Turbill, C. & Ruf, T. Senescence is more important in the natural lives of long- than short-lived mammals. PLoS ONE 5, e12019 (2010).
Van Essen, D. Organization of visual areas in macaque and human cerebral cortex. In The Visual Neurosciences Volume 1. eds. Chalupa, L. M. & Werner, J. S. (Cambridge MA: MIT Press, 2004). 507-521.
Van Essen, D. C., Anderson, C. H. & Felleman, D. J. Information processing in the primate visual system: an integrated systems perspective. Science 255, 419-423 (1992).
van Schaik, C. P. & Deaner, R. O. Life history and cognitive evolution in primates. In Animal Social Complexity. eds. de Waal, F. B. M. & Tyack, P. L. (Cambridge MA: Harvard University Press, 2003). 5-25.
Veilleux, C. C. & Kirk, E. C. Visual acuity in the cathemeral strepsirrhine Eulemur macaco flavifrons. American Journal of Primatology 71, 1-10 (2009).
Walls, G. L. The Vertebrate Eye and Its Adaptive Radiation. New York: Hafner Publishing Company (1942).