Abstract
Study design:
Prospective vasopressor cross-over interventional study
Objectives:
To examine how two vasopressors used in acute traumatic spinal cord injury (SCI) affect intrathecal cerebrospinal fluid pressure and the corresponding spinal cord perfusion pressure (SCPP).
Setting:
Vancouver, British Columbia, Canada.
Methods:
Acute SCI patients over the age of 17 with cervical or thoracic ASIA Impairment Scale (AIS). A, B or C injuries were enrolled in this study. Two vasopressors, norepinephrine and dopamine, were evaluated in a ‘crossover procedure’ to directly compare their effect on the intrathecal pressure (ITP). The vasopressor cross-over procedures were performed in the intensive care unit where ITP, mean arterial pressure (MAP) and heart rate were being continuously measured. The SCPP was calculated as the difference between MAP and ITP.
Results:
A total of 11 patients were enrolled and included in our analysis. There were 6 patients with AIS A, 3 with AIS B and 2 with AIS C injuries at baseline. We performed 24 cross-over interventions in these 11 patients. There was no difference in MAP with the use of norepinephrine versus dopamine (84±1 mm Hg for both; P=0.33). Conversely, ITP was significantly lower with the use of norepinephrine than with dopamine (17±1 mm Hg vs 20±1 mm Hg, respectively, P<0.001). This decrease in ITP with norepinephrine resulted in an increased SCPP during the norepinephrine infusion when compared with dopamine (67±1 mm Hg vs 65±1 mm Hg respectively, P=0.0049).
Conclusion:
Norepinephrine was able to maintain MAP with a lower ITP and a correspondingly higher SCPP as compared with dopamine in this study. These results suggest that norepinephrine may be preferable to dopamine if vasopressor support is required post SCI to maintain elevated MAPs in accordance with published guidelines.
Similar content being viewed by others
Introduction
Following the primary mechanical trauma of acute spinal cord injury (SCI), a number of injury mechanisms are triggered, which may contribute to further secondary damage.1, 2 Of the many postulated pathomechanisms of secondary injury, ischemia is thought to have particular significance. Local changes within the traumatically injured spinal cord that contribute to ischemia include the direct disruption of microvasculature and the impairment of autoregulation.1 Systemic hypotension and diminished cardiac output can further reduce spinal cord perfusion and exacerbate the ischemic insult.3
Ischemia has practical importance as a mechanism of secondary injury because it may be directly influenced by systemic blood pressure and hemodynamic management during the acute injury period. Uncontrolled observational studies examining protocols of care in acute SCI, including blood pressure management, recommended maintaining a minimum mean arterial pressure (MAP) of 85–90 mm Hg.4, 5 Many subsequent literature reviews and guidelines have concluded that hypotension should be avoided in acute SCI patients, and MAP should be maintained at around 85–90 mm Hg for a period of about 1 week post injury.6, 7, 8 Recently, Hawryluk et al.9 reported in a retrospective cohort of 74 acute SCI patients with MAP recordings performed every minute that increased MAP correlated with improved neurologic recovery and, importantly, that hypotension was associated with poor recovery.
The ultimate goal of increasing the MAP, and as a result, increasing spinal cord perfusion pressure (SCPP), is to augment spinal cord blood flow. In traumatic brain injury, cerebral perfusion pressure is calculated as the difference between MAP and intracranial pressure. Similarly, SCPP is calculated as the difference between the MAP and intrathecal cerebrospinal fluid (CSF) pressure.10 Previously, we reported that the CSF pressure—as measured through indwelling lumbar intrathecal catheters—increased unexpectedly during the first 3–4 days post injury in acute SCI patients.11 With a constant MAP, an increase in CSF pressure reduces SCPP. This decrease in SCPP would otherwise go undetected without intrathecal pressure (ITP) monitoring. In our previous study, we observed increases of 10–15 mm Hg of CSF pressure during continuous monitoring in some of our patients. Similarly, elegant work by Papadopoulos and colleagues12 has shown significant increases in ITP at the injury site as a result of cord swelling against the dura.10
The elevation of MAP for acute SCI may be achieved by intravenous volume replacement or administration of vasopressor medications, the latter of which are routinely used to achieve a desired MAP target.9 A number of different vasopressors are currently used in clinical practice, including phenylephrine, dopamine and norepinephrine. Comparisons of the cerebral neurovascular effects of different vasopressors have been reported in the clinical traumatic brain injury literature.13, 14, 15 However, such comparisons of the neurovascular effects of different vasopressors are lacking in acute human SCI, although their differing rates of complications have been reported.16, 17 The choice of vasopressor is typically dictated largely by physician and/or institutional preference.
In our current prospective cross-over interventional study, we examined how two vasopressors (norepinephrine and dopamine) affected ITP (as measured by an intrathecal lumbar catheter) and the corresponding SCPP.
Materials and methods
The acute SCI patients who were evaluated in this study were part of an ongoing prospective observational trial at our institution, in which lumbar intrathecal catheters were inserted to monitor ITP, and then simultaneous monitoring of MAP and ITP was conducted for 3–5 days post injury (ClinicalTrials.gov NCT01279811). The clinical trial protocol including the procedures for testing norepinephrine and dopamine received approval from our institutional Clinical Research Ethics Board (REB #H10-01091). All patients provided verbal and/or written informed consent to be included in the clinical study.
Acute SCI patients over the age of 17 with cervical or thoracic ASIA Impairment Scale (AIS) A, B or C injuries were enrolled in this study. The main inclusion criteria were the ability to conduct a valid baseline neurologic examination according to ISNCSCI standards, obtain an informed consent from the patient and insert a lumbar catheter within 48 h from the time of injury. Patients who sustained concomitant brain injury (traumatic and non-traumatic), severe multi-trauma, intoxication/sedation or other major injuries that precluded a reliable neurologic examination were excluded.
Intrathecal catheter insertion was performed preoperatively under the supervision of a spine surgeon.10, 18 Under sterile conditions, a lumbar intrathecal catheter (PERIFIX Custom Epidural Anesthesia Kit, B. Braun Medical Inc, Bethleham, PA, USA) was inserted at L2/3 or L3/4 and advanced 15–20 cm from its entry point. The catheter was then connected to a Becker External Drainage and Monitoring System (Medtronic, Inc., Minneapolis, MN, USA). The monitoring system’s pressure transducer was zeroed at the level of the patient’s right atrium, approximated clinically at the patient’s midaxillary line. The transducer was recalibrated whenever there was a change to the patient’s bed height or inclination. For MAP monitoring, a radial artery catheter was placed. The transducers for the ITP and arterial line were connected to a multichannel monitoring system (SpaceLabs Healthcare, Issaquah, WA, USA) to allow for simultaneous display of ITP and MAP.
Two vasopressors, norepinephrine and dopamine, were evaluated in a ‘cross-over procedure’ to directly compare their effect on ITP. The vasopressor cross-over procedures were performed in the intensive care unit where the ITP, MAP and heart rate were being continuously measured. The target MAP in the intensive care unit for these patients was between 80 and 85 mm Hg. At our institution, we typically aim for a MAP in this region instead of aggressively pursuing a MAP at or above 90 mm Hg with vasopressors. The procedures began with the recording of MAP, heart rate and ITP for 15 min to obtain a stable ‘baseline’. For patients whose MAP was being maintained on norepinephrine, dopamine was started (at 1–20 mcg kg−1 min−1) and titrated up to maintain the baseline MAP, whereas norepinephrine was weaned off over 5–10 min. The MAP was then maintained on dopamine alone for 30 min, after which the norepinephrine was recommenced and the dopamine weaned off over 5–10 min. For patients whose MAP was being maintained on dopamine, the same procedure was carried out by switching them over to norepinephrine alone and then back to dopamine. The ITP, MAP and heart rate values were continuously monitored and recorded for another 15 min after ‘crossing back’ to the original vasopressor. The SCPP was calculated as the difference between MAP and ITP. The vasopressor cross-over procedures were conducted during times when the patient was able to rest quietly for the entire duration of the procedure (that is, not undergoing physiotherapy or other nursing interventions).
We present the data using median and range, or mean and s.d., for non-normally and normally distributed data, respectively. We used Stata 10.0 (StataCorp, College Station, TX, USA) for analysis. We used paired t-tests to compare the changes in MAP, ITP and SCPP after dopamine and norepinephrine interventions. All tests were two sided, and a P-value of <0.05 was considered statistically significant. The sample size was one of convenience and represented the number of patients who we could recruit with the available resources.
Results
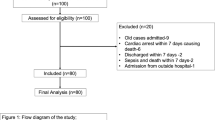
A total of 11 patients were enrolled and included in our analysis. The cohort included 10 subjects with cervical injuries and 1 subject with a thoracic injury. The median age of the patients was 38 years (range: 17–60 years). There were 6 patients with AIS A, 3 with AIS B and 2 with AIS C injuries at baseline. Demographic details of the patients enrolled in the study are shown in Table 1.
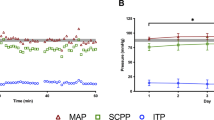
Most commonly, the patients were being maintained on norepinephrine (22 of the 24 cross-over interventions) and then were crossed over to dopamine. There were two cross-over interventions where the maintenance vasopressor was dopamine, which was crossed over to norepinephrine. Some patients complained of discomfort and palpitations while on the dopamine and the cross-over interventions in these individuals were terminated. In the end, we were able to conduct 24 cross-over interventions in 11 patients. The average MAP, SCPP and ITP during each of these cross-over procedures are listed in Table 2. The differences between these three parameters while on dopamine or norepinephrine are illustrated in Figure 1. There was no difference in average MAP during the infusions of dopamine (84±1 mm Hg) and norepinephrine (84±1 mm Hg; P=0.33), indicating that, during the cross-over procedure, we were able to compare changes in the ITP independent of the MAP (Figure 1a). In contrast, ITP was significantly lower with the use of norepinephrine than with dopamine (17±1 mm Hg vs 20±1 mm Hg, respectively, P<0.001; Figure 1b). This decrease in ITP with norepinephrine resulted in an increased SCPP during the norepinephrine infusion when compared with dopamine (67±1 mm Hg vs 65±1 mm Hg respectively, P=0.0049; Figure 1c). Typically, the switching of the patient from norepinephrine to dopamine would result in an almost immediate rise in the ITP, which then reverted quickly back to the original level when switched back to the norepinephrine. An illustrative example of this is shown in Figure 2.
Connected line plots comparing effects of dopamine and norepinephrine on MAP (a), ITP (b) and SCPP (c) in mm Hg. The gray lines represent a change in pressure in an individual patient. The black line is the mean change in pressure for the overall cohort. ITP was ~3 mm Hg lower and SCPP was ~2 mm Hg higher on norepinephrine compared with dopamine (P<0.001 and P=0.0049, respectively).
Rapid changes in ITP during vasopressor cross-over. This is an illustrative example of the rapid changes in ITP that were observed when switching a patient from norepinephrine to dopamine. Notice as the norepinephrine is dropped and the dopamine is introduced, there is an immediate increase in ITP of about 5 mm Hg. This reverts back to baseline when the dopamine is stopped and the norepinephrine is re-introduced.
Discussion
In this prospective, single-center cross-over study comparing norepinephrine to dopamine in patients with acute SCI, we demonstrated that dopamine was associated with increased ITP when compared with norepinephrine. The increase in ITP occurred despite a stable MAP and thus resulted in decreased SCPP with dopamine use compared with norepinephrine.
Although current guidelines recommend maintaining a MAP of 85 to 90 mm Hg following acute SCI,19 there is no formal recommendation regarding which vasopressor to use to achieve this target. The decision about which specific vasopressor to use in acute neurotrauma is typically left to the discretion of the treating physician, whose choice may be based on desired pharmacologic activity, personal familiarity/experience and institutional bias/tradition. This has resulted in considerable variations in clinical practice in the use of vasopressors in the TBI and SCI populations. For example, a retrospective study of pediatric TBI patients at a single institution reported that vasopressor usage varied widely, with the first-line treatment most commonly being phenylephrine (57%), followed by dopamine (29%), norepinephrine (10%) and epinephrine (4%).20 In a series of 114 adult TBI patients, phenylephrine was reportedly the most commonly used vasopressor (43%), followed by norepinephrine (30%), dopamine (22%) and vasopressin (5%).15 In a series of acute SCI patients, dopamine was reportedly the most commonly used vasopressor (48%), followed by phenylephrine (45%), norepinephrine (5.0%) epinephrine (1.5%) and vasopressin (0.5%).16 In contrast, at our local institution, the initial vasopressor of choice for acute SCI is typically norepinephrine, followed by dopamine. These observations simply highlight the equipoise that exists with respect to vasopressor usage in acute SCI.
This equipoise is interesting because, although all of these vasopressors are effective at augmenting MAP, they each have unique pharmacologic properties, based on their profile for agonism of α-adrenergic, β-adrenergic, dopamine and vasopressin receptors. As such, it is reasonable to expect that they would have different effects on perfusion within the injured central nervous system.21 Norepinephrine is a potent α-adrenergic agonist with less-pronounced β-adrenergic agonist effects, increasing MAP primarily by vasoconstriction but also potentially by increasing cardiac output. Dopamine has dose-dependent effects, with dopaminergic effects at low doses and mixed α- and β-adrenergic effects at higher doses, causing vasoconstriction and increased cardiac output.
Although little is known about the differential effects of vasopressors within the injured spinal cord, studies of cerebral blood flow and metabolic responses certainly support the notion that different vasopressors have different effects on cerebral perfusion after injury.22 For example, some studies that have compared norepinephrine and dopamine in animal models of TBI have reported better cerebral perfusion around the injury penumbra with norepinephrine23, 24 and the potential for worsened vasogenic edema with the use of dopamine.25 In a cross-over study comparing dopamine and norepinephrine in 19 patients admitted with a severe TBI, dopamine resulted in increased ICP and resultant decrease in CPP, when compared with norepinephrine.13 In a randomized, cross-over study of TBI patients, norepinephrine resulted in more consistent increases in transcranial Doppler cerebral blood flow velocity when compared with dopamine.14 An additional clinical TBI study reported that, in contrast to dopamine, norepinephrine consistently increased global and regional cerebral oxygenation and decreased the regional oxygen extraction fraction and ischemic brain volume in TBI.26 Clearly, there is room for further study on the use of such vasopressors, particularly in the setting of traumatic SCI.
In the absence of a method for directly measuring blood flow or oxygenation within the injured spinal cord, we performed this experimental study to ask the question of whether norepinephrine and dopamine had differential effects on ITP in acute SCI patients. We addressed this question in this manner because we had the opportunity to continuously measure MAP and ITP simultaneously and could evaluate ITP while using either vasopressor to maintain a constant MAP.
We were able to maintain MAP above our target threshold of 80–85 mm Hg during the majority of the study. However, MAP can be dynamic and consistent maintenance of MAP thresholds can by challenging in the setting of traumatic brain injury27 or SCI.28 Although there was variability in the MAP values achieved, the average MAP achieved during the cross-over interventions was essentially the same between dopamine and norepinephrine (mean of 84 mm Hg). This allowed us to study the effects of the two vasopressors on ITP and SCPP during our study independent of the influence of significant fluctuations in the MAP.
After an acute traumatic SCI, the injured section of the spinal cord becomes swollen, resulting in reduced amount of CSF between the neural tissue and the surrounding dura mater. Papadopoulos and colleagues12 have elegantly demonstrated that intraspinal pressure at the injury site was higher than pressure simultaneously recorded from the CSF compartment below or extradurally.11 In the presence of a swollen spinal cord with a reduced surrounding CSF space, any further increase in the CSF pressure may worsen SCPP and exacerbate secondary injury to the spinal cord. Our study suggests that norepinephrine results in lower ITP and improved SCPP as compared with dopamine post injury. We were surprised at first to see how rapidly the ITP increased when we switched the patients from norepinephrine to dopamine, and how similarly quickly it decreased when switching the patients back to norepinephrine (as shown in Figure 2).
The difference in mean SCPP between norepinephrine and dopamine was only about 2 mm Hg, and whether such a difference in SCPP is clinically significant is unclear. Given that both vasopressors can be used to increase MAP, the slightly better SCPP with norepinephrine would suggest that there is at least a theoretical perfusion advantage to this vasopressor. Also, in the recent work of Hawryluk et al.,9 in which almost 1 million minute-by-minute MAP recordings were made in 74 acute SCI patients, there was a strong association between average MAP during the first 3 days post injury. In the analysis of all 74 patients with outcome data, the patients who did not achieve AIS grade improvement had an average MAP of ~93 mm Hg, and those who had 1 grade or more than 1 grade improvement had average MAPs of ~94 and 96 mm Hg, respectively. This would suggest that even small changes in MAP may indeed be clinically significant.
Varsos et al.29 recently described his experimental findings of worsening of spinal autoregulation at both low and high SCPP. He found that, when spinal autoregulatory capacity was maintained, there was an inverse relationship between changes in arterial blood pressure and CSF pressure. When spinal autoregulatory capacity was disrupted, there was found to be a direct relationship between the arterial blood pressure and the CSF pressure, where any increase in arterial blood pressure will result in an increase in the CSF pressure also. It may be that the increased ITP seen with dopamine may reflect dysfunctional autoregulation given that MAP was maintained constant. The effects of dopamine on autoregulation have not been studied in patients with SCI but are conflicting in other populations. A similar vasopressor cross-over study was conducted on patients with traumatic brain injury by Ract and Vigué.13 Using continuous hemodynamic and ICP monitoring, these investigators also ‘swapped’ epinephrine and dopamine in severe TBI patients while keeping MAP constant. The authors reported that ICP was also significantly higher with dopamine than with norepinephrine and that this increased ICP may be related to a vasodilatory effect of dopamine.13 Dopamine has been associated with impaired cerebral autoregulation in preterm infants as measured by near infrared spectroscopy30 and with preserved cerebral autoregulation in a pig model of traumatic brain injury.31 Further studies are required to understand the precise mechanisms accounting for this difference in the setting of SCI.
An obvious limitation of this current study is that it was not designed nor powered to determine whether one vasopressor was associated with better neurologic recovery than another. This was purely an experimental study to examine how ITP was affected by two different vasopressors. We are therefore left to speculate on the clinical importance of a 2 mm Hg improvement in SCPP. Although 2 mm Hg seems like a rather small difference, such a small difference in average MAP was indeed relevant for predicting AIS conversion in the work of Hawryluk et al.9 Regardless of the clinical significance, if there is indeed equipoise in the selection of vasopressors, but a potential perfusion advantage exists with one agent (norepinephrine) over another (dopamine), then such a finding may be important when selecting a vasopressor agent. If a physician is comfortable with and willing to use either drug to raise MAP in the acutely injured patient, then there may be a little downside to choosing the agent that might provide better perfusion, even if this difference is small. Another limitation of this study is that, although phenylephrine is also a commonly used vasopressor in SCI, this was not included in our study because of institutional preferences.
Ten of the eleven patients in this study sustained cervical injuries with only one patient with a thoracic injury. It is well-known that cervical SCIs are more commonly associated with a significant loss of sympathetic input as compared with thoracic and lower level SCI. The differential physiological effects from SCI at the level of the cervical spine as compared with SCI at lower levels may limit the generalizabilty of our findings to those patients with cervical SCI only.
In conclusion, we found that the choice of vasopressor does have an effect on ITP independent of the MAP, thereby influencing SCPP. Norepinephrine was able to maintain MAP with a lower ITP and a correspondingly higher SCPP as compared with dopamine in this study. These results suggest that norepinephrine may be preferable to dopamine if vasopressor support is required post SCI to maintain elevated MAPs in accordance with published guidelines. This recommendation is reinforced with recently published literature describing a higher complication rate with the use of dopamine in the intensive care setting.16, 17 Further study comparing neurologic outcomes using different vasopressors would obviously be desirable, but we acknowledge that such a study would be exceedingly challenging to conduct in the acute SCI population. We contend, however, that investigating these subtle differences that may occur with standard treatment decisions will help physicians in their efforts to optimize the hemodynamic management of acute SCI patients.
Data archiving
There were no data to deposit.
References
Tator CH, Fehlings MG . Review of the secondary injury theory of acute spinal cord trauma with emphasis on vascular mechanisms. J Neurosurg 1991; 75: 15–26.
Dumont RJ, Verma S, Okonkwo DO, Hurlbert RJ, Boulos PT, Ellegala DB et al. Acute spinal cord injury, part I: pathophysiologic mechanisms. Clin Neuropharmacol 2001; 24: 254–264.
Tator CH . Experimental and clinical studies of the pathophysiology and management of acute spinal cord injury. J Spinal Cord Med 1996; 19: 206–214.
Vale FL, Burns J, Jackson AB, Hadley MN . Combined medical and surgical treatment after acute spinal cord injury: results of a prospective pilot study to assess the merits of aggressive medical resuscitation and blood pressure management. J Neurosurg 1997; 87: 239–246.
Levi L, Wolf A, Belzberg H . Hemodynamic parameters in patients with acute cervical cord trauma: description, intervention, and prediction of outcome. Neurosurgery 1993; 33: 1007–1016.
Ryken CT, Hurlbert RJ, Hadley MN, Aarabi B, Dhall SS, Gelb DE et al. The acute cardiopulmonary management of patients with cervical spinal cord injuries. Neurosurgery 2013; 72: 84–92.
Casha S, Christie S . A systematic review of intensive cardiopulmonary management after spinal cord injury. J Neurotrauma 2011; 28: 1479–1495.
Consortium for Spinal Cord Medicine. Early acute management in adults with spinal cord injury: a clinical practice guideline for health-care professionals. J Spinal Cord Med 2008; 31: 403–479.
Hawryluk G, Whetstone W, Saigal R, Ferguson A, Talbott J, Bresnahan J et al. Mean arterial blood pressure correlates with neurological recovery after human spinal cord injury: analysis of high frequency physiologic data. J Neurotrauma 2015; 32: 1958–1967.
Kwon BK, Curt A, Belanger LM, Bernardo A, Chan D, Markez JA et al. Intrathecal pressure monitoring and cerebrospinal fluid draining in acute spinal cord injury: a prospective randomized trial. J Neurosurg Spine 2009; 10: 181–193.
Werndle MC, Saadoun S, Phang I, Czosnyka M, Varsos GV, Czosnyka ZH et al. Monitoring of spinal cord perfusion pressure in acute spinal cord injury: initial findings of the injured spinal cord pressure evaluation study. Crit Care Med 2014; 42: 646–655.
Phang I, Papadopoulos MC . Intraspinal pressure monitoring in a patient with spinal cord injury reveals different intradural compartments: Injured Spinal Cord Pressure Evaluation (ISCoPE) Study. Neurocritical Care 2015; 23: 414–418.
Ract C, Vigué B . Comparison of the cerebral effects of dopamine and norepinephrine in severely head-injured patients. Intensive Care Med 2001; 27: 101–106.
Steiner LA, Johnston AJ, Czosnyka M, Chatfield DA, Salvador R, Coles JP et al. Direct comparison of cerebrovascular effects of norepinephrine and dopamine in head-injured patients. Crit Care Med 2004; 32: 1049–1054.
Sookplung P, Siriussawakul A, Malakouti A, Sharma D, Wang J, Souter MJ et al. Vasopressor use and effect on blood pressure after severe adult traumatic brain injury. Neurocrit Care 2011; 15: 46–54.
Inoue T, Manley GT, Patel N, Whetstone WD . Medical and surgical management after spinal cord injury: vasopressor usage, early surgerys, and complications. J Neurotrauma 2014; 31: 284–291.
Readdy WJ, Whetstone WD, Ferguson AR, Talbott JF, Inoue T, Saigal R et al. Complications and outcomes of vasopressor usage in acute traumatic central cord syndrome. J Neurosurg Spine, (e-pub ahead of print 31 July 2015).
Kwon BK, Stammers AM, Belanger LM, Bernardo A, Chan D, Bishop CM et al. Cerebrospinal fluid inflammatory cytokines and biomarkers of injury severity in acute human spinal cord injury. J Neurotrauma 2010; 27: 669–682.
Hadley M, Walters B, Grabb P, Oyesiku NM, Przybylski GJ, Resnick DK et al. Guidelines for the management of acute cervical spine and spinal cord injuries. Clin Neurosurg 2002; 49: 407–498.
Di Gennaro JL, Mack CD, Malakouti A, Zimmerman JJ, Armstead W, Vavilala MS . Use and effect of vasopressors after pediatric traumatic brain injury. Dev Neurosci 2010; 32: 420–430.
Muzevich KM, Voils SA . Role of vasopressor administration in patients with acute neurologic injury. Neurocrit Care 2009; 11: 112–119.
Pfister D, Strebel SP, Steiner LA . Effects of catecholamines on cerebral blood vessels in patients with traumatic brain injury. Eur J Anaesthesiol Suppl 2008; 42: 98–103.
Kroppenstedt SN, Sakowitz OW, Thomale UW, Unterberg AW, Stover JF . Influence of norepinephrine and dopamine on cortical perfusion, EEG activity, extracellular glutamate, and brain edema in rats after controlled cortical impact injury. J Neurotrauma 2002; 19: 1421–1432.
Kroppenstedt SN, Sakowitz OW, Thomale UW, Unterberg AW, Stover JF . Norepinephrine is superior to dopamine in increasing cortical perfusion following controlled cortical impact injury in rats. Acta Neurochir Suppl 2002; 81: 225–227.
Beaumont A, Hayasaki K, Marmarou A, Barzo P, Fatouros P, Corwin F . The effects of dopamine on edema formation in two models of traumatic brain injury. Acta Neurochir Suppl 2000; 76: 147–151.
Johnston AJ, Steiner LA, Chatfield DA, Coles JP, Hutchinson PJ, Al-Rawi PG et al. Effect of cerebral perfusion pressure augmentation with dopamine and norepinephrine on global and focal brain oxygenation after traumatic brain injury. Intensive Care Med 2004; 30: 791–797.
Clifton GL, Choi SC, Miller ER, Levin HS, Smith KR Jr, Muizelaar JP et al. Intercenter variance in clinical trials of head trauma-experience of the National Acute Brain Injury Study: hypothermia. J Neurosurg 2001; 95: 751–755.
Kong CY, Hosseini AM, Belanger LM, Ronco JJ, Paquette SJ, Boyd MC et al. A prospective evaluation of hemodynamic management in acute spinal cord injury patients. Spinal Cord 2013; 51: 466–471.
Varsos GV, Werndle MC, Czosnyka ZH, Smielewski P, Kolias AG, Phang I et al. Intraspinal pressure and spinal cord perfusion pressure after spinal cord injury: an observational study. J Neurosurg Spine 2015; 14: 1–9.
Eriksen VR, Hahn GH, Greisen G . Dopamine therapy is associated with impaired cerebral autoregulation in preterm infants. Acta Paediatr 2014; 103: 1221–1226.
Armstead WM, Riley J, Vavilala MS . Dopamine prevents impairment of autoregulation after traumatic brain injury in the newborn pig through inhibition of up-regulation of endothelin-1 and extracellular signal-regulated kinase mitogen-activated protein kinase. Pediatr Crit Care Med 2013; 14: e103–e111.
Acknowledgements
We acknowledge the research staff of the Vancouver Spine Research Program, in particular Allan Aludino, Leilani Reichl, Angela Tsang and Jiwan Gill for their assistance with this study. We also acknowledge support for the conduct of this study from the Michael Smith Foundation for Health Research, the Craig H. Neilsen Foundation and the Rick Hansen Institute (RHI), including Daniel Rogers of RHI for project management. Dr Griesdale is supported by the VGH & UBC Hospital Foundation Best of Health Fund. MFD holds the Paetzold Chair in Spinal Cord Injury Research. FKK holds the Canada Research Chair in Spinal Cord Injury and VGH & UBC Hospital Foundation Dvorak Chair in Spine Trauma.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Altaf, F., Griesdale, D., Belanger, L. et al. The differential effects of norepinephrine and dopamine on cerebrospinal fluid pressure and spinal cord perfusion pressure after acute human spinal cord injury. Spinal Cord 55, 33–38 (2017). https://doi.org/10.1038/sc.2016.79
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sc.2016.79
This article is cited by
-
Pharmacologic and Acute Management of Spinal Cord Injury in Adults and Children
Current Treatment Options in Neurology (2022)
-
Neurorestorative interventions involving bioelectronic implants after spinal cord injury
Bioelectronic Medicine (2019)
-
Modern Medical Management of Spinal Cord Injury
Current Neurology and Neuroscience Reports (2019)
-
Clinical Trials in Traumatic Spinal Cord Injury
Neurotherapeutics (2018)