Abstract
Study design:
Case report
Background/objective:
Myasthenia gravis (MG) complicating spinal cord injury (SCI) is extremely rare. We report a patient with SCI developing MG leading to death. There are no similar articles at present on literature search.
Case report:
A 54-year-old man, paralysed at the T12 level (ASIA A) for 40 years, was admitted for surgical repair of his grade IV sacral pressure sore. During the admission he developed diplopia, fluctuating dysphagia and slurred speech. Elevated anti-acetylcholine receptor antibodies and single fibre electromyography confirmed the diagnosis of MG and pyridostigmine was commenced. His admission was complicated by intermittent episodes of unexplained tachycardia and tachypnoea. He succumbed following cardio respiratory within 6 weeks of admission. Post mortem examination was inconclusive of a definite cause of death. In the presence of SCI, it can be challenging to diagnose MG or its complications like myasthenic and cholinergic crisis.
Conclusion:
The case highlights the difficulty in diagnosis and management of MG in persons with SCI.
Similar content being viewed by others
Introduction
Myasthenia gravis (MG) is a disorder of neuromuscular transmission caused by auto antibodies against nicotinic acetylcholine receptor or muscle specific tyrosine kinase.1 It affects around 100 per million and is more common in women, male to female ratio being 2:3.2 This disease is characterised by fluctuating weakness of skeletal muscles with fatigability and recovery after a period of rest. Extra ocular muscles, bulbar muscles and muscles of respiration are frequently affected. The disease can cause respiratory failure requiring ventilation. The diagnostic tests are high titres of acetyl choline receptor antibodies, repetitive nerve stimulation test showing decremental response, single fibre electromyography showing increased jitter and blocking and objective relief of signs and symptoms on intravenous administration of edrophonium, a short active acetyl choline esterase inhibitor.1 Treatments of MG are symptomatic treatment with cholinesterase inhibitors like pyridostigmine and definitive treatment with immunosuppression using corticosteroids or drugs like azathioprine or mycophenolate.3
Clinical symptoms and signs of MG are exclusively motor. In people with traumatic spinal cord injury (SCI) presence of weakness below the level of injury makes diagnosis of MG difficult. SCI and its complications like urinary sepsis can also exacerbate MG.
Case report
A 54-year-old man, paralysed at the T12 level (ASIA A) for 40 years after fall from a tree. He was admitted for surgical repair of his grade IV sacral pressure sore.
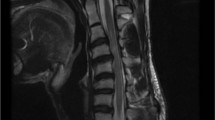
While in the hospital he reported slurring of speech and headache. Two weeks later he developed diplopia. His neurological examination was normal except for subjective double vision, which improved after eye closure and worsened on sustained lateral gaze. His magnetic resonance imaging of the brain was normal. Magnetic resonance imaging of the spine did not show any evidence of spinal cord compression or syringomyelia. Computed tomography scan of the thorax did not show any evidence of thymoma.
In view of the symptoms, ocular myasthenia was considered. Repetitive nerve stimulation test was normal. Stimulated single fibre electromyography showed increased jitter with blocking consistent with myasthenia. His anti-acetylcholine receptor antibody titres were 30 nmol l−1 (normal range 0–0.2 nmol l−1) consistent with diagnosis of myasthenia. He was started on pyridostigmine and atropine and his ocular symptoms improved. Five days later, he died suddenly following an episode of tachycardia. His arterial blood gases and oxygen saturations were normal. His autopsy did not show any obvious cause of death.
Discussion
Our report is the first report of MG in a patient with SCI. Motor weakness due to SCI can alter clinical signs and symptoms of MG. Common presentations of MG are fatigable weakness of the extra ocular muscles, muscles used in swallowing and limb muscles. As people with SCI already have limb weakness, clinical suspicion of MG need to be based on weakness of extra ocular muscles or swallowing.
Investigations in MG are anti-acetylcholine receptor antibodies, repetitive nerve stimulation test and single fibre electromyography. High titres of anti-acetylcholine receptor antibody were found in 85% of people with generalised MG and 50% of people with ocular MG.4 Presence of this antibody is very specific for MG and false positive results are very low. Our patient had very high titres of this antibody, which is very specific for MG. In MG, repetitive nerve stimulation at low frequencies (1–5 Hz) will cause a reduction in size of compound muscle action potentials. This test was negative in our subject. Cui et al. reported that repetitive nerve stimulation test was positive in only 27.8% of people with ocular MG.5 A negative repetitive nerve stimulation test does not exclude MG.6 In single fibre electromyography, a needle electrode record potentials from a single motor unit. In MG, interval between action potentials from two or more single muscle fibres from same motor unit muscle action potentials (jitter) will increase. This is a more sensitive and specific test than repetitive nerve stimulation and is indicated in people with suspected MG and negative repetitive nerve stimulation test.6
Our patient died suddenly and autopsy was inconclusive. Acute exacerbation of weakness in a patient on treatment for MG could be a myasthenic crisis or a cholinergic crisis. Myasthenic crisis is a medical emergency due to worsening MG and may lead to respiratory failure. Cholinergic crisis results from excess anti-cholinesterase inhibitors. There is excess acetylcholine stimulation of the striated muscle producing motor symptoms indistinguishable from myasthenic crisis. The signs differentiating cholinergic crisis from myasthenic crisis include miosis, salivation, lacrimation, urinary in continence, diarrhoea, gastric upset and emesis.1 Our patient did not have clinical features of either cholinergic or myasthenic crisis.
Both MG and traumatic SCI are relatively uncommon disorders. Lin et al. reported a patient whose MG worsened after traumatic SCI.7 Several drugs including amino glycosides, corticosteroids and succinyl choline can worsen MG.1 People with SCI are also at risk of infections like urinary tract infections and pressure ulcers, which in turn can exacerbate MG. These factors need to be considered while caring for a person with MG and SCI.
Conclusion
The case highlights the difficulty in diagnosis and management of MG and its complications in people with SCI. SCI or complications related to it like urinary sepsis can precipitate myasthenic crisis. Further with the background of complete paraplegia it is difficult to diagnose fluctuating generalised weakness, which most patients with MG commonly have apart from bulbar symptoms.
References
Jacon S, Viegas S, Lashley D, Hilton-Jones D . Myasthenia gravis and other neuromuscular junction disorders. Pract Neurol 2009; 9: 364–371.
Phillips LH . The epidemiologyof myasthenia gravis. Semin Neurol 2004; 24: 17–20.
Skeie GO, Apostolski S, Evoli A, Gilhus NE, Illa I, Harms L et al. Guidelines for treatment of autoimmune neuromuscular transmission disorders. Eur J Neurol 2010; 17: 893–902.
Meriggiolo MN . Myasthenia gravis with anti-acetyl choline receptor antibodies. Front Neurol Neurosci 2009; 26: 94–108.
Cui LY, Guan YZ, Wang H, Tang XF . Single fiber electromyography in the diagnosis of ocular myasthenia gravis: report of 90 cases. Chin Med J (Engl) 2004; 117: 848–851.
Srivastava A, Kalita J, Misra UK . A comparative study of single fibre electromyography and repetitive nerve stimulation in consecutive patients with myasthenia gravis. Electromyogr Clin Neurophysiol 2007; 47: 93–96.
Lin CS, Wang JH, Wang YH, Pan SL . Myasthenia Gravis with superimposed spinal cord injury: a case report. J Rehabil Med 2008; 40: 684–686.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Kolli, S., Mathew, K., Thumbikat, P. et al. Superimposed myasthenia gravis in chronic spinal cord injury: a case report. Spinal Cord 49, 1206–1207 (2011). https://doi.org/10.1038/sc.2010.182
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sc.2010.182
Keywords
This article is cited by
-
Ocular myasthenia gravis in a person with tetraplegia presenting challenges in diagnosis and management
Spinal Cord Series and Cases (2016)