Abstract
Study Design: Case Report.
Objective: Thrombolytic therapy has become a routine and valuable care for selected patients with acute myocardial infarction (AMI) and rarely complicates with spinal epidural hemorrhage causing cord compression.
Setting: Elazig, Turkey
Case report: A 72-year-old woman developed spinal epidural hemorrhage following streptokinase and heparin administration for AMI. Back pain and lower extremity neurologic deficits ensued secondary to spinal cord compression by epidural hematoma. Diagnosis of spinal epidural hematoma, extending through T11 to L2 vertebra levels, could be accurately made by magnetic resonance imaging (MRI). Careful follow-up by neurologic examination, reversal of anticoagulant effects, anti-edema treatment with steroids and a low-intensity rehabilitation program maintained a full recovery. Follow-up MRI, 3 months after the accident, revealed complete resolution of the hematoma.
Conclusion: Physicians should be aware of this rare complication secondary to thrombolytic therapy. A high index of suspicion for hemorrhagic complications is necessary, particularly in elderly patients under thrombolytic treatment regardless of spinal pain, and the patient's lethargic or confused status should be taken into account. MRI is a valuable imaging option that gives information on both localization and extent of lesion and recovery.
Similar content being viewed by others
Introduction
During the past decade, numerous large clinical trials have shown that thrombolytic agents are the cornerstone of treatment for patients with acute myocardial infarction (AMI), and impressive reductions in mortality associated with the use of these agents in the setting of AMI have been achieved.1,2 However, thrombolysis is related to bleeding complications, particularly intracranial hemorrhage that has the most disastrous consequences and is associated with high mortality rate.1,2 Intraspinal hemorrhage complicating thrombolysis for myocardial infarction (MI) is a rare entity but important due to its acute and progressive course, which can lead to debilitating neurological deficits and even death.3,4
We report a case of spontaneous spinal epidural hemorrhage following administration of streptokinase for AMI.
Case report
A 72-year-old woman without prior cardiac history was admitted to the emergency department of our institution with profuse sweating, left arm and crushing substernal chest pain continuing for 2–3 h. Blood pressure was 160/120 mmHg and pulse was 86 beats/min. Her physical examination was unremarkable. She was on calcitonin 200 IU/day and calcium treatment for osteoporosis. There was no history of bleeding, tobacco or alcohol use. Acute posterior-lateral myocardial infarction was diagnosed on the basis of electrocardiogram findings and confirmed by elevation of cardiac enzymes. Treatment was started with aspirin, nitroglycerin and 5000 U heparin. Streptokinase was given 1 500 000 U intravenously. Approximately 48 h after streptokinase administration, she complained of weakness and numbness in both her legs. She also complained of increase in her dorsolumbar pain, which she had been experienced for years.
Neurological examination revealed an awake and oriented patient. Her upper extremity muscle strength was 5/5 with normal-active reflexes. The right lower extremity strength was 1/5 and the left lower extremity strength was 2/5, and both lower extremities were flaccid. Deep tendon reflexes were abolished and plantar reflexes were equivocal. There was bilateral loss of pinprick sensation below L2, and bilateral loss of perception. Her anal reflex was intact. She developed urinary retention and was catheterized. Her prothrombin time was 11.2 s, and activated partial thromboplastin time was 65.2 s.
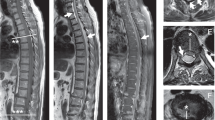
Magnetic resonance imaging (MRI) was carried out at the third day. MRI revealed posterior epidural hematoma, causing slight cord compression, hyperintense in T1-weighted, heterogeneous-hyperintense in T2-weighted images, extending through T11 to L2 vertebra levels (Figure 1a,b).
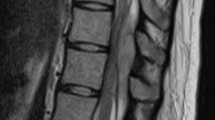
Heparin was discontinued and vitamin K was administered. Dexamethasone 16 mg/day (4 mg every 6 h intravenously) was administered. On follow-up, the patient's neurologic deficits had not deteriorated. Within 2 days, sensory findings gradually improved and the patient was able to move her left lower extremity. Owing to the gradual improvement in neurological findings and the patient's prohibitive cardiovascular status, an operation was not performed. The patient continued active-assistive range of motion exercises, and underwent a low-intensity rehabilitation program 3 months after the accident. Follow-up MRI, 3 months after the hemorrhage, revealed complete resolution of the hematoma (Figure 2).
At the last visit 1 year after the hemorrhagic event, the patient had made a full neurologic recovery and was independent in daily living activities.
Discussion
The thrombolytic agents, including streptokinase and urokinase, have systemic effects, and recombinant tissue-type plasminogen activator (tPA), a fibrin selective agent, alters homeostasis through the activation of plasminogen. The efficacy of thrombolytic therapy in decreasing mortality from AMI has been shown in a broad group of patients.1,2 However, bleeding complications occur varying 5–40% in reported series, and the most serious complication, intracranial hemorrhage, occurs in 0.46–0.88% of patients treated with these thrombolytic agents.1,2 On the other hand, intraspinal hemorrhage complicating thrombolytic agents is extremely rare. Anticoagulation therapy with heparin or warfarin, accompanying with other causes as hemophilia and hepatic dysfunction, accounts for most of the intraspinal hemorrhages.3,5
The factors triggering or contributing to the development of spinal, epi- or subdural hemorrhage with spinal compression may be listed as trauma, coagulation disorders (anticoagulants, bleeding disorders, blood dyscrasia), vascular malformations, spinal anesthesia, tumors, infections, vasculitic syndromes, and arterial hypertension, atherosclerosis or alcohol abuse.3,4,5 Spinal epidural hemorrhages are rare and usually occur idiopathically (52%) but are sometimes associated with coagulopathies (25%).4
The most frequent clinical symptom of a spinal epidural hematoma is neck or back pain with or without radicular symptoms. Neurological deficits from spinal cord compression can appear insidiously or abruptly and progress in a wide clinical picture of complete paraplegia/tetraplegia to Brown–Séquard's syndrome.3,4,5,6 It is important to clinicians to be aware of the signs and symptoms that sometimes could not be expressed by confused or lethargic patients who were under the effect of medications. Published reports presenting painless spinal hemorrhages support this notion.7,8 Also the differential diagnosis of epi- or subdural hematoma includes: pathologic fractures related to metastases, primary tumors or osteoporosis; intervertebral disc diseases; transverse myelitis and Guillain Barré syndrome, anterior spinal artery syndrome; spinal vascular malformations; spinal infections particularly epidural abscess formation; and spondylitis.3,4,6,9,10,11,12,13
MRI scan is the investigation of choice for suspected epidural hematoma. Nearly two decades earlier, in the pre-MRI period, diagnosis was usually based on myelography or CT myelography. MRI gives multiplanar accurate information on both the location and extent of hematoma, as well as the intensity of spinal cord compression by the lesion.10 MRI also gives information about the age of hematoma, and is useful in differentiating accompanying intraspinal masses and to follow-up the resolution of the hematoma. However, application of MRI is prohibited in patients with stainless-steel stent implantation. However, some of the stents allow the use of magnetic resonance.4 In our case MRI was undertaken since our patient did not have coronary dilatation and stent implantation. In cases with stainless-steel implants, computed tomography with or without myelography could be used as an imaging technique.
In a recent report, 10 cases of spinal epidural hematoma associated with thrombolytic treatment have been reviewed.4 TPA treatment was the leading thrombolytic agent causing epidural hemorrhage alone or in combination with heparin. Streptokinase-related epidural hematoma has been previously reported by Mustafa and Gallino11 in a 49-year-old man following administration of streptokinase and heparin for AMI. This patient reported by Mustafa and Gallino11 underwent lumbar laminectomy. Another case of streptokinase-related intrinsic spinal cord hemorrhage has been reported by Cruickshank et al,13 in which the patient died within a few days after streptokinase administration.
On the basis of reviewed case reports, Baron et al4 suggested that marked elevation of the partial thromboplastin time or fibrin products or combined use of heparin with thrombolytic agents may increase the risk of bleeding. As in our patient, it may also be considered that increased fragility of vascular structures, atherosclerosis and dysfunctional platelets in elderly patients may be responsible for the increased risk of hemorrhagic complications after thrombolysis.4,14
Emergency surgery is the treatment for this condition but patient-specific factors should be carefully evaluated prior to adopting surgery. Anticoagulants and thrombolytics should be immediately discontinued and correction of coagulopathy should be undertaken prior to surgery. As in our case, the patient's cardiovascular status may be prohibitive for surgery. In such cases, reversal of coagulant effects, close observation of neurologic deficits and in occasional cases, methylprednisolone administration may achieve good results without surgery.6,9
Spinal epidural hemorrhage secondary to thrombolytic therapy is a rare complication that consulting physicians should be aware of. A high index of suspicion for hemorrhagic complications is necessary in elderly patients under thrombolytic treatment regardless of spinal pain, and the patient's lethargic or confused status should be taken into account. MRI is a valuable imaging option that gives information on both localization and extent of lesion and recovery.
References
Gurwitz JH et al. Risk for intracranial hemorrhage after tissue plasminogen activator treatment for acute myocardial infarction. Ann Intern Med 1998; 129: 597–604.
Sloan MA et al. Clinical features and pathogenesis of intracerebral hemorrhage after rt-PA and heparin therapy for acute myocardial infarction: the thrombolysis in myocardial infarction (TIMI) II pilot and randomized clinical trial combined experience. Neurology 1995; 45: 649–658.
Kreppel D, Antoniadis G, Seeling W . Spinal hematoma: a literature survey with a meta-analysis of 613 patients. Neurosurg Rev 2003; 26: 1–49.
Baron EM, Burke JA, Akhtar N, Young WF . Spinal epidural hematoma associated with tissue plasminogen activator treatment of acute myocardial infarction. Cathet Cardiovasc Intervent 1999; 48: 390–396.
Sawin PD, Traynelis VC, Follett KA . Spinal epidural hematoma following coronary thrombolysis with tissue plasminogen activator. J Neurosurg 1995; 83: 350–353.
Connoly SE, Winfree CJ, McCormick PC . Management of spinal epidural hematoma after tissue plasminogen activator. A case report. Spine 1996; 21: 1694–1698.
Cohen JE, Howard JG, Emery D, Schwartz ML . Fatal spontaneous spinal epidural hematoma following thrombolysis for myocardial infarction. Surg Neurol 1998; 49: 520–523.
Smith RF, Bodin CJ, Kogutt MS . Recent epidural anesthesia: a relative contraindication to thrombolysis. Am J Roentgenol 1997; 169: 445–446.
Lopez AG, Lara JMP, Hidalgo RH, Gonzalo PE . Spinal epidural hematoma following thrombolytic therapy for acute myocardial infarction. Orthopedics 1999; 22: 987–988.
van Heesewijk JPM, Caparie JWBM . Acute spontaneous spinal epidural hematoma in a child. Eur Radiol 2000; 10: 1874–1876.
Mustafa MH, Gallino R . Spontaneous spinal epidural hematoma causing cord compression after streptokinase and heparin therapy for acute coronary artery acclusion. South Med J 1988; 81: 1202–1203.
Duyur B, Erdem HR, Ozgocmen S . Paravertebral abscess formation and knee arthritis due to Brucellosis in a patient with rheumatoid arthritis. Spinal Cord 2001; 39: 554–556.
Cruickshank GS, Duncan R, Hadley DM, Bone I . Intrinsic spinal cord heamorrhage due to streptokinase treatment for myocardial infarction. J Neurol Neurosurg Psychiatr 1992; 55: 740.
Serebruany VL et al. Depressed platelet status in an elderly patient with hemorrhagic stroke after thrombolysis for acute myocardial infarction. Stroke 1998; 29: 235–238.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Ozgocmen, S., Yoldas, T., Kocakoc, E. et al. Spinal epidural hematoma associated with streptokinase treatment for myocardial infarction. Spinal Cord 42, 374–377 (2004). https://doi.org/10.1038/sj.sc.3101604
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.sc.3101604