Abstract
“Smart Materials” are materials that change their shape, color, or size in response to an externally applied stimulus. While smart materials have already made a tremendous impact on our lives through their applications in liquid crystal displays, headphones, fuel injection systems, flexible cell phone antennas, and many other commercial products, they also have the potential to help many pediatric patients. This review focuses on with the present and potential applications of shape memory alloys, piezoelectric materials, and the relatively newer class of materials called magnetostrictive and ferromagnetic shape memory alloys in the design of pediatric cardiovascular devices.
Similar content being viewed by others
Main
“Smart Materials” are materials that change their shape, color, or size in response to an externally applied stimulus. Examples of “external stimuli” include changes in temperature, application of an electric or magnetic field, and changes in moisture or pH. Thus, whereas other materials play passive structural roles in devices, smart materials can actually be manipulated in situ.
During the last few decades smart materials have had a tremendous impact on a variety of fields. For example, chromogenic materials are the basis of all liquid crystal displays and photochromes allow eyeglasses to change to sunglasses in the presence of sun light. The basis of lighter igniter switches, headphones, and recent fuel injection systems is piezoelectrics whereas the flexible cell phone antennas and eyeglasses are shape memory smart materials. Many more commercial applications for smart materials are already available and new ones are being studied by a wide range of organizations.
This review focuses on with the potential applications of shape memory alloys, piezoelectric materials, and the relatively newer class of materials called magnetostrictive and ferromagnetic shape memory alloys (FSMA) in the design of pediatric cardiovascular devices. Whereas shape memory alloys like nitinol (a nickel-titanium alloy) change shape in response to a change in temperature, magnetostrictive or ferromagnetic materials change their shape in response to a magnetic field. NiMnGa (nickel-manganese-gallium) and Terfenol-D represent the best known examples of FSMA and magnetostrictive materials respectively. The more mature class of piezoelectric materials, e.g., BaTiO3 and lead zirconate titanate (Pb(ZrTi)O3)—commonly called PZT, change shape with an electrical field; or, conversely, generate an electrical signal in response to a forced change in shape. All of these materials can be used as either an actuator or a sensor.
Smart materials have begun to have a significant impact in the field of medicine. Although a number of applications already exist, it is only a matter of time before many more pediatric applications will emerge for these smart materials.
SHAPE MEMORY ALLOYS
Nitinol introduction.
NiTi or nitinol is a nickel-titanium shape memory alloy. Nitinol is a pneumonic for Nickel-Titanium, developed by the Naval Ordinance Laboratory in the early 1960s by Buehler. Although initial applications for shape memory and psuedoelastic NiTi were in industrial applications, it was later found to be biologically inert. This later discovery, coupled with its psuedoelastic response has made it ideal for use in a variety of medical applications. Specifically, it is ideal for transcatheter use where device deployment is the primary objective. The fairly unique thermal/mechanical induced phase transformation from martensite to austenite (or the reverse) gives rise to nitinol's shape memory and psuedoelastic capabilities. In the low temperature martensite phase (monoclinic state), nitinol is very malleable due to reversible twin boundary motion (unlike dislocation motion in most metals) and can be compressed into catheters. Upon heating (in many cases simply to body temperature), nitinol transforms to its parent phase, austenite which has a cubic crystal structure.
When undergoing the phase transformation to the cubic or austenite state, nitinol recovers its original shape and eliminates the deformation induced in the martensite state. This original shape can be set into the material by simply heat treating the material in a constrained shape near its crystallization temperature (500 C) for a few minutes. Therefore, nitinol can be “taught” to recover virtually any shape as long as the deformations are below 10%. For comparisons, other competitive elastic materials such as surgical steel and Ti are limited to 1% elastic deformations. Thus, nitinol can be compressed into physical dimensions substantially smaller than other competitive elastic materials while still elastically recovering its original shape. In addition to these unique structural properties, nitinol has been found to be biocompatible.
Nitinol is also known to be resistant to thrombus formation and does not calcify (1–9). A thin titanium oxide layer forms on nitinol's surface, similar to that formed on Ti, and prevents corrosion or reaction of the bulk material in physiologic solutions. Although the long term effects of titanium oxide in vivo have not been extensively studied, it is possible that the layer of titanium oxide which forms on NiTi devices in the vascular system could prevent extensively leaching of nickel into the body. Allergic reactions to NiTi devices placed in the cardiovascular system are certainly possible. If they do occur, these reactions are certainly very rare and may not occur. Long term studies of NiTi devices placed in the vasculature certainly will need to be done to assess for allergic reactions as well as leaching of nickel and long term effects on the local tissue. To date, no Amplatzer NiTi cardiovascular device has needed to be explanted because of an allergic reaction (personal communication with AGA Medical, Golden Valley, MN).
NiTi stents.
The most obvious and widely used application of nitinol has been in the design of stents for stenotic or blocked blood vessels. “Self-expanding” stents are made from nitinol. These stents can be cooled and compressed into a sheath outside the body when nitinol is in its martensite phase (martensite NiTi is very malleable). Once positioned appropriately inside the body, these stents are heated to body temperature, they transition from martensite to austenite and when they are extruded from their external sheath within the body, they spring open to the fully expanded shape that they were trained to be in their austenite form (Fig. 1). Nitinol stents have allowed a select group of patients to avoid surgery. In pediatrics, self-expanding stents have found niche applications for stenting of the patent ductus arteriosus in cyanotic patients with congenital heart disease (10–12).
NiTi closure devices.
Bulk nitinol has also been used for the design of pediatric devices designed to close holes in the heart [atrial septal defects (ASDs) and ventricular septal defects (VSDs)] as well as the patent ductus arteriosus (PDA). As with stents, shape memory alloys are ideally suited for use in this sort of device because they can be “trained” to form certain shapes at body temperature, while also capable of being cooled to the martensite shape and compressed into very small catheters.
The ASD, patent foramen ovale (PFO), VSD and PDA nitinol closure devices manufactured by AGA Medical (Golden Valley, MN) were originally designed by Kurt Amplatz, M.D. (a radiologist). These devices have significantly improved the treatment of these basic congenital heart defects. All of the devices are built from mesh tubes of woven NiTi wires (the NiTi wires used are as small as 0.003 inches in diameter) and are shape set to form whatever general shapes are needed to close a given congenital heart defect. For example, the Amplatzer Septal Occluder for ASD closures forms two disks with a waist in between and can be easily delivered so that it is stable even in a beating heart. The ASO devices can be delivered in sheathes as small as 6 Fr and range in sizes up to devices appropriate for closure of 35 mm to 4 cm ASDs (the larger devices require up to a 12 Fr sheath; see Fig. 1) (3,12). Most of the Amplatzer devices are designed such that the heart can adapt to the shape of the device.
ASD, VSD, PFO, and PDA closure devices have allowed over 50,000 patients with congenital heart disease to undergo nonsurgical closure of these significant congenital heart defects. In addition to AGA Medical, many other companies have now used a range of different designs for ASD, PFO, and PDA closure devices. Nearly all of these devices use nitinol's unique shape memory properties (13–18). Of note, a nitinol coil manufactured by pfm Medical (Cologne, Germany) is now being used for closure of both PDAs and smaller VSDs (19).
Thin film NiTi.
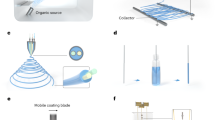
Thin films are defined as being about 1 to 15 μm thick—more than “paper thin.” (For reference purposes hair is nominally 50 μm thick.) During the last two decades researchers have attempted to fabricate thin film Nitinol with marginal success. The manufacturing of thin film nitinol has been attempted by vacuum evaporation, flash evaporation, ion beam sputtering, and laser ablation (20–25). Most of these fabrication approaches have been unsuccessful at producing quality thin film required in medical applications. Vacuum sputter deposition, which involves knocking atoms from a target material and directing them to form a thin film on a substrate in an extremely low pressure vacuum (Fig. 2), has been used in attempts to produce high quality thin film nitinol. This approach, for the time being, is the generally preferred method for thin film fabrication because of process controllability and consistency. Because sputter deposition “nanosynthesis” requires an ultrapure vacuum chamber (less than 10−12 torr) there are very few contaminants introduced during the fabrication process.
Although the general process of sputter deposition of various materials has been successfully used by many industries, only a handful of teams have been able to produce high quality thin films of nitinol using a sputter deposition approach (18–21). Recently, our group discovered that target heating during the sputtering process creates uniform films not present in conventional sputtering processes (26). Developing sufficient experience with this particular process produces fairly precise control of the film composition as well as uniformity during the sputter deposition technique. The target composition and annealing temperatures (27) needed to produce nitinol thin films with transition temperatures in the desired temperature range for medical applications has also been determined. These advancements should facilitate the introduction and subsequent use of thin film NiTi in biomedical devices.
Because thin film NiTi with predictable transition temperatures can be produced with sputter deposition, this process can now be used to produce thin film NiTi for biomedical applications. Several groups and companies including ABPS (Advanced Bioprosthetic Surfaces, San Antonio, TX) are now actively developing medical devices with thin film nitinol.
Thin film NiTi transcatheter heart valves.
Thin film nitinol has favorable biologic properties as well as elastic properties, which make it an ideal material for use in transcatheter heart valves. Because nitinol also has shape memory, entire heart valves (the leaflets and support structure) can be made entirely from nitinol such that they can be compressed into a catheter and delivered into the heart without using cardiopulmonary bypass and without an invasive surgical procedure.
A thin film nitinol heart valve could represent the ideal hybrid valve: it could have the biologic compatibility and nonthrombogenicity of tissue valves and the longevity of a mechanical valve (nitinol has very impressive fatigue properties). Although tissue valves are now being placed percutaneously in both the aortic and pulmonary positions, none of the currently available transcatheter valves have leaflets with shape memory, none have the longevity of a mechanical valve and all of the valves currently in trial require sheaths >18–19 Fr. Transcatheter valves which incorporate thin film nitinol would certainly have a lower profile (less than even 5 Fr), would have shape memory leaflets and would likely have very favorable fatigue properties (if designed correctly).
A team led by Julio Palmaz, M.D., Steven Bailey, M.D., and Chris Banos at ABPS has designed and has begun animal testing on a semilunar thin film NiTi heart valve. The ABPS valve incorporates leaflets made from 10 μm membranes of thin film nitinol, which is made to be very flexible by incorporation of multiple regularly spaced small fenestrations. These membranes termed “eNitinol” are sufficiently flexible to be used as leaflets in a semilunar valve design. The leaflets are designed to serve has very flexible scaffoldings for the growth of endothelial cells—this essentially converts this entirely metal valve into a tissue valve through a normal biologic response. The ABPS “eNitinol membrane PercValve” has an elegantly designed supporting scaffolding which enables it to be self expandable. The ABPS heart valve can collapse into a 10 Fr sheath, and a similar valve designed for use in the venous system can be delivered through a 4.8 Fr sheath (Fig. 3).
Subcoronary implantations of the self-expanding ABPS PercValve have been accomplished in animal studies. The valves have been shown to have very low opening pressures, and endothelialize completely by 10 d. As would be expected, these thin film NiTi valves certainly do not calcify and are very low-profile—especially when compared with the transcatheter valves currently in human trials. Although testing is currently in progress, these valves could have very unique advantages in sealing of the valve annulus and for improved valve durability. Because they endothelialize rapidly, they are certainly biologically friendly. They could certainly be made low profile enough for use in even small infants (<5 Fr).
Currently, the utility of thin film NiTi in developing percutaneously placed heart valves for use in the pediatric population is also being investigated in the UCLA Active Materials Laboratory. Our group has used thin film NiTi for the construction of a heart valve, which is currently in laboratory and animal testing (27). We found that nonfenestrated thin film nitinol was too stiff for use in a semilunar valve. Thus, we used nonfenestrated thin film nitinol to construct a bileaflet valves from semicircular pieces of thin film NiTi. This valve is more similar in design to other bioprosthetic mechanical valves, but is also designed to endothelize quickly for improved function. However, its design has made it much more difficult to collapse when compared with the ABPS valve.
Thin film NiTi applications: covered stents.
Although traditional stents are nonocclusive metal scaffoldings, the treatment of many disease processes relies on the ability to use a “covered stent,” able to both open vessels and provide a circumferentially occlusive boundary between the stent and the vessel. Covered stents are used for stenting vessels that are at risk for rupturing (or have already ruptured), are aneurysmal, or are at high risk of in-stent stenosis. The next generation of stents could be significantly improved by using thin film NiTi materials: not only could thin film nitinol improve the biologic response to a stent, but it can allow for the production of very low profile covered stents appropriate even for use in small neonates.
While studying the use of thin films for transcatheter valves, our group also began to examine the preliminary biocompatibility of thin film NiTi covered stents (Fig. 4). Not only did the thin film NiTi appear to prevent intimal proliferation into the stent, but it appeared to promote endothelialization which was detectable by scanning electron microscopy as early as 14 d. Longer term studies of professionally engineered thin film NiTi stents in both the venous, coronary, and CNS vasculature are certainly justified.
With covered stents in general there is a fear that stents will be difficult to remove surgically or redilate. Unpublished data from our lab has shown that covered stents can be redilated to accommodate exponential growth of swine but that high-pressure balloons are needed. We have also shown that stents covered with thin film nitinol are exceedingly easier to dissect from the surrounding native tissue as compared with uncovered stents.
Thin film nitinol could allow for the production of very low profile neurovascular self-expanding stents appropriate for even newborns. It could also aid in the design and production of a low profile (less than 8–10F) aortic stent graft. Although thin film stent applications will certainly be seen first in adult patients, many niche applications will become available in pediatric patients. These applications include stenting of the PDA in cyanotic newborns with congenital heart disease (28), treatment of arteriovenous malformations and pulmonary vein stenting. These applications are probably more suited to thin film Nitinol due to the fact that thin film can be collapsed into much smaller diameter catheters than “bulk” materials, i.e., smaller catheters are a requirement in the pediatric community.
Future of thin film NiTi.
There is the potential for a vast realm of future thin film NiTi in many other pediatric biomedical/clinical applications. Thin film nitinol's shape memory behavior can also be used to do work. Our group has manufactured fluid pumps from thin film NiTi and demonstrated that their power production per unit volume is substantially higher than other small scale pumps. It would be possible to use a thin film NiTi pump has a ventricular assist device (VAD) for children with heart failure or for children with a single ventricle and a failing Fontan.
FERROMAGNETIC SHAPE MEMORY ALLOYS
With the application of a magnetic field, nickel-manganese-gallium and other (FSMAs) generate strains of up to 10%. These strains result from the action of twin boundary motion rather than from the phase transformation observed in traditional shape memory alloys such as nitinol. This mechanism also differs from traditional magnetostrictive materials (i.e., Terfenol-D) that generate strains of only 0.2% through magnetic domain wall motion.
Magnetic field induced strain (MFIS) in FSMAs was first observed in Ni2MnGa in 1996 when researchers measured 0.2% strain in fields of 640 kA/m (29). By 2000, researchers reported MFIS of 6% in single crystal samples of Ni49.8Mn28.5Ga21.5 and, in 2002, strains approaching 10% were reported in single crystal samples of Ni48.8Mn29.7Ga21.7 (30–32). The variable degree of magnetically induced strain in these samples is attributed to differences in the NiMnGa crystal structure. The dimensions of the crystal lattice dictate the maximum obtainable MFIS while magnetic anisotropy dictate the maximum force output (31–32).
The strain output generated in NiMnGa is comparable to commercially available shape memory alloys (such as NiTi) commonly used for the design of stents and other biomedical devices. Unlike nitinol, NiMnGa can be operated with the application of a magnetic field and as such can be actuated from a remote site (e.g., outside the body). As opposed to complicated cyclic actuations, NiMnGa and other FSMA materials are ideally suited for one-way actuation. For a stent design, the NiMnGa sample would initially exist in a compressed formation and not require a catheter to hold it in this position, thus potentially reducing the size of the delivery system. Once in the body, a magnetic field would be applied to return it to its fully expanded configuration. Thus, use of NiMnGa for the design of a stent would not require cyclic loading, two-way actuation/shape-memory, or a reset mechanism. It remains to be seen if FSMA stents can generate adequate radial force at diameters as high as 12–28 mm.
Since the initial discovery of large magnetically induced strain in FSMAs, a substantial amount of experimental research has focused on characterization of the material properties of these materials. However, to date, few applications exist and many potential uses are limited by the large magnetic fields required to actuate the material's relatively low output pressures. However, as standard magnetic resonance imaging scanners generate up to 4 Tesla, substantially more than necessary to transform NiMnGa (i.e., less than 1 Tesla), these problems will not prevent this material's use in biomedical applications. Additionally, the output pressures required in medical applications are on the order of kPa—well below the 2–4 MPa limit of NiMnGa.
A transcatheter NiMnGa device (a stent, occluder or trigger for another device) would allow for deployment without a delivery sheath and without a balloon. Magnetically activated FSMA stents could allow physicians to easily open stenosis and treat aneurysms in remote vessels now consider very difficult or impossible to access. There would be immediate applications of this technology in interventional neuroradiology (stenting of intracranial vessels and occlusions of CNS aneurysms) as well as in peripheral and coronary applications. Given the appropriate biocompatibility and efficacy, FSMA stents could potentially replace all current stent technology and would benefit the broad range of patients with peripheral vascular disease, cardiovascular disease, neurovascular disease and congenital heart disease.
Preliminary work has also been performed to characterize NiMnGa crystals in the UCLA Active Materials laboratory. MFIS of over 4% were consistently generated and the effect of tensile load on MFIS was characterized (33,34). To date, however, no devices containing NiMnGa have ever been implanted in a human or in an animal. Medical devices made from NiMnGa would be completely novel and could allow for the beginning of magnetically activated transcatheter devices. While NiMnGa is a promising material, unfortunately, NiMnGa is unlikely to be resistant to corrosion. Although, its poor resistance to biocorrosion could be the primary factor which limits its cardiovascular applications in children, this may be circumvented in the future with coating technologies or the development of new FSMA materials.
PIEZOELECTRIC MATERIALS
Introduction.
An array of piezoelectric materials comprises the functional component of the modern day ultrasound and echocardiograph probes. Their unparalleled ability to act as a transducer between voltage and pressure at elevated frequency makes them highly useful for biomedical imaging. However, the application of piezoelectric materials in pediatrics is likely to extend well past imaging applications.
The piezoelectric effect was discovered in the late 19th century by Pierre and Jacques Curie. Their joint knowledge of crystallography and pyroelectricity—the ability of a material to generate current in response to temperature stimuli—enabled them to effectively demonstrate the property of quartz and other selected minerals to generate electric potential under mechanical stress. In the early 20th century, German physicist, Woldemar Voigt, published a text—Lehrbuch der Kristallphysik—describing the piezoelectric effect and the classes of naturally-occurring crystals capable of producing it.
Piezoelectric materials are characterized by the ability to generate electric potential in response to applied stress. Conversely, piezoelectric materials deform upon the application of a potential. The piezoelectric effect relies on the asymmetric charge distribution within individual crystalline units. Upon application of a mechanical stress, the deforming units displace the positive and negative charges with respect to one another. This displacement produces a change in polarization of the crystal that creates a measurable electrical potential. Materials exhibiting the piezoelectric effect include a variety of naturally occurring crystals, natural materials, manufactured crystals, manufactured ceramics, and special man-made polymers. However, certain materials are far more efficient than others in generating larger voltages under smaller stress changes.
Manufactured crystals are generally modeled after naturally occurring formations – this is the case with the quartz analogues gallium orthophosphate (GaPO4) and langasite (La3Ga5SiO14). Select manufactured ceramics such as lithium niobate (LiNbO3) and perhaps the most common piezoelectric ceramic, PZT also display the piezoelectric effect. Finally, some polymers exhibit the piezoelectric effect. Polymers such as polyvinylidene fluoride deform to give the induced attractive and repulsive forces. Piezoelectric polymers are widely used in low-frequency single-element sensor systems due to their relatively high piezoelectric stress coefficient. They are also useful in some acoustic applications where mechanical impedance matching is important, i.e., polymers are an order of magnitude lower stiffness than ceramic piezoelectric materials.
Early applications harnessed the ability of piezoelectric devices to act as transducers between electric potential and frequency. The first practical application of piezoelectric devices was the development of sonar during World War I. Langevin produced a transducer composed of thin quartz crystals sandwiched between steel plates. Ultrasound was generated by the piezoelectric device and subsequently detected by an underwater microphone. Based on the time for the ultrasonic pulse to be detected, spatial distances could accurately be determined. Further research extended the field of piezoelectrics into time-domain reflectometers, voltage generation, frequency standardization, systemic stabilization, sensing, and actuation. Piezoelectric generators are now being studied to harvest energy, serve as a frequency standard, voltage rectification systems, and to counteract unwanted vibration in dynamic systems.
Ultrasonic imaging.
Piezoelectric ultrasound was first used to detect flaws in metals in the 1920s, and was then used to assess mitral valve disease in the1950s. Today, the echocardiogram is the gold standard for the rapid evaluation of the heart muscle and valves. In vivo, endocardial piezoelectric crystals have been implanted along different axes of the heart and to detect altered left ventricular-arterial coupling which precedes pump dysfunction in early heart failure (35). Today surgeons use transducer-tipped catheters in ultrasound biomicroscopy, and in the future it is reasonable to expect that piezotechnology technology will yield a complete virtual heart display based on ultrasonic imaging (36). Finally, a developing piezo-based sensor is being designed to render heart sounds into a visual image. Although significant progress still needs to be made (37), piezo-based sensors could certainly make the stethoscope into a more visual and quantitative tool.
Piezoelectric sensors have also emerged as a noninvasive method of providing warning of impending failure in prosthetic heart valves secondary to leaflet degradation or thrombus formation. Sensors mounted onto the housing of mechanical valves create an electrical signal secondary to loading from thrombi or the valve's own vibrations (38). Using data from piezo-sensors on heart valves, physicians can optimize postimplantation therapies such as anti-coagulants and can detect leaflet dysfunction.
A new method to detect cardiac ischemia utilizes a 3-axis piezoelectric accelerometer sutured via surgical procedure onto the lateral free wall of the left ventricle. In an occluded area, there is a noticeable change to stronger power of higher harmonics of the signal. Early recognition of regional cardiac ischemia is possible through analysis of the accelerometer signal real-time via the fast Fourier_ transform (39). Although the primary application of this device will be in adults vulnerable to cardiac ischemia, it could also have tremendous implications for monitoring of pediatric patients after heart surgery or cardiac transplantation.
Piezoelectric cardiac assistance devices and micropumps for drug delivery.
Piezoelectric devices are very efficient motors and are very easy to miniaturize; thus, they are ideally suited for use as implantable drug pumps (i.e., as needed in diabetes and potentially for chronic delivery of inotropes to patients with cardiomyopathies). A prototype peristaltic piezoelectric micropump system has been described and manufactured (40,41). This piezoelectric micropump can provide a constant flow rate over a wide backpressure range, enabling high-resolution volumetric dosing with adjustable stroke volume and power minimization. Because PZT micro-pumps can displace exact quantities of very small volumes, they can be used to delivery precise volumes of high concentration drugs to the systemic circulation (42).
Because of their efficiency and size, piezoelectric materials have also been used to build cardiac assist devices or VADs. These devices are used to pump blood in series or in parallel with the native heart in the setting of extreme hear failure (as from a cardiomyopathy). Piezo-VADs have now been tested in vitro and in vivo.
One piezoelectric VAD consists of two cantilevers, each a piezoelectric bender element, which are synchronized with the paced native heartbeat. The benders are loaded with high density masses to produce resonant frequencies matching the heart rate of the subject. These benders subsequently compress thin-walled tubing carrying blood in an oscillatory fashion. Testing of this device in a simulated circulatory system revealed a desirable insensitivity to spatial orientation or changing mean fluid pressure (43). The device has the advantages of simple construction, lower power consumption than earlier devices, and (instead of pneumatic drive) noiseless electrical operation. Additionally, piezoelectrics consume only 5% of the power required by pneumatic systems and require no invasive pneumatic tubing (43).
Another ventricular assist device contains a differential piezoresistive pressure sensor within an intracardiac pump catheter as part of a sophisticated self-management system. The piezoelectric device monitors the pressures during pump operation and provides feedback to the intracardiac pump allowing for somewhat autonomous biventricular support (44).
Piezoelectric materials have potential application in actuation as well as biomedical imaging and sensing. Collectively, modern piezo-based ventricular assistance devices constitute the next leap toward constructing an artificial heart. Using piezoelectrics to harvest energy from the heart could allow pacemakers (and even ventricular assist devices) to work indefinitely without battery changes.
CONCLUSIONS
Smart materials have an amazing potential to impact the care of the pediatric patient. Many applications of smart materials in pediatric devices are already being developed and many others are in development. Even the early stage applications of shape-memory alloys, magnetostrictive materials and piezoelectrics for pediatric cardiovascular devices may begin to make a huge impact on our patients in the next decade.
Abbreviations
- ASD:
-
atrial septal defect
- FSMA:
-
ferromagnetic shape memory alloys
- MFIS:
-
magnetic field induced strain
- NiMnGa:
-
Nickel Manganese Gallium
- NiTi:
-
nitinol
- PDA:
-
patent ductus arteriosus
- PFO:
-
patent foramen ovale
- PZT:
-
lead zirconate titanate (Pb(ZrTi)O3)
- VSD:
-
ventricular septal defect
References
Armitage DA, Grant DM 2003 Characterisation of surface-modified nickel titanium alloys. Mater Sci Eng 349: 89–97
Armitage DA, Parker TL, Grant DM 2003 Biocompatibility and hemocompatibility of surface-modified NiTi alloys. J Biomed Mater Res A 66: 129–137
Ryhanen J, Niemi E, Serlo W, Niemela E, Sandvik P, Pernu H, Salo T 1997 Biocompatibility of nickel-titanium shape memory metal and its corrosion behavior in human cell cultures. J Biomed Mater Res 35: 451–457
Trepanier C, Leung T, Tabrizian M, Yahia L, Bienvenu J, Tanguay J, Piron D, Bilodeau L 1999 Preliminary investigation of the effects of surface treatments on biological response to shape memory NiTi stents. J Biomed Mater Res 48: 165–171
Weaver DJ, Veldhuizen AG, Sanders MM, Schakenraad JM, van Horn JR 1997 Cytotoxic, allergic and genotoxic activity of a nickel-titanium alloy. Biomaterials 18: 1115–1120
Tepe G, Wendel HP, Khorchidi S, Schmehl J, Wiskirchen J, Pusich B, Claussen CD, Duda SH 2002 Thrombogenicity of various endovascular stent types: an in vitro evaluation. J Vasc Interv Radiol 13: 1029–1035
Thierry B, Merhi Y, Bilodeau L, Trépanier C, Tabrizian M 2002 Nitinol versus stainless steel stents: acute thrombogenicity study in an ex vivo porcine model. Biomaterials 23: 2997–3005
Rocher P, El Medawar L, Hornez JC, Traisnel M, Breme J, Hildenbrand HF 2004 Biocorrosion and cytocompatibility assessment of NiTi shape memory alloys. Scr Mater 50: 255–260
Shabalovskaya SA 2002 Surface, corrosion and biocompatibility aspects of nitinol as an implant. Biomed Mater Eng 12: 69–109
Dundar Y, Hill RA, Bakhai A, Dickson R, Walley T 2004 Angioplasty and stents in coronary artery disease: a systematic review and meta-analysis. Scand Cardiovasc J 38: 200–210
Rabe K, Sievert H 2004 Carotid artery stenting. J Interv Cardiol 17: 417–426
Cheatham JP 2001 Stenting of coarctation of the aorta. Catheter Cardiovasc Interv 54: 112–125
Thanopoulos BV, Rigby ML, Karanasios E, Stefanadis C, Blom N, Ottenkamp J, Zarayelyan A 2007 Transcatheter closure of perimembranous ventricular septal defects in infants and children using the amplatzer perimembranous ventricular septal defect occluder. Am J Cardiol 99: 984–989
Pass RH, Hijazi Z, Hsu DT, Lewis V, Hellenbrand WE 2004 Multicenter USA amplatzer patent ductus arteriosus occlusion device trial: initial and one-year results. J Am Coll Cardiol 44: 513–519
Du ZD, Hijazi ZM, Kleinman CS, Silverman NH, Larntz K, Amplatzer Investigators 2002 Comparison between transcatheter and surgical closure of secundum atrial septal defect in children and adults: results of a multicenter nonrandomized trial. J Am Coll Cardiol 39: 1836–1844
Chessa M, Carminati M, Cao QL, Butera G, Giusti S, Bini RM, Hijazi ZM 2002 Transcatheter closure of congenital and acquired muscular ventricular septal defects using the amplatzer device. J Invasive Cardiol 14: 322–327
Hong TE, Thaler D, Brorson J, Heitschmidt M, Hijazi ZM, Amplatzer PFO Investigators 2003 Transcatheter closure of patent foramen ovale associated with paradoxical embolism using the Amplatzer PFO occluder: initial and intermediate-term results of the US multicenter clinical trial. Catheter Cardiovasc Interv 60: 524–528
Chan KC, Godman MJ, Walsh K, Wilson N, Redington A, Gibbs JL 1999 Transcatheter closure of atrial septal defect and interatrial communications with a new self expanding nitinol double disc device (amplatzer septal occluder): multicentre UK experience. Heart 82: 300–306
Lorber A, Gazit AZ, Khoury A, Schwartz Y, Freudental F 2003 Percutaneous transaortic occlusion of patent ductus arteriosus using a new versatile angiographic and delivery catheter. Pediatr Cardiol 24: 482–483
Busch JD, Johnson AD, Lee CH, Stevenson DA 1990 Shape-memory properties in Ni-Ti sputter-deposited film. J Appl Phys 68: 6224–6228
Sekiguchi Y, Funami K, Funakubo H 1983 Deposition of NiTi shape memory alloy thin film by vacuum evaporation. Proceedings of 32nd Meeting of Japan Society of Materials, 65–67.
Makino E, Uenoyama M, Shibata T 1998 Flash evaporation of TiNi shape memory thin film for microactuators. Sensor Actuat A 71: 187–192
Lehnert T, Tixier S, Boni P, Gotthardt R 1999 A new fabrication process for Ni–Ti shape memory thin films. Mater Sci Eng A 273– 275: 713–716
Ikuta K, Hayashi M, Matsuura T, Fujishiro H 1994 An investigation of micro structures, sensors, actuators, machines and robotic systems. Proceedings of the IEEE Workshop Micro Electro Mechanical Systems, Oiso, Japan, pp 355–360.
Ming H Wu 2001 Fabrication of nitinol materials and components. Proceedings of the International Conference on Shape Memory and Superelastic Technologies, Kunming, China, pp 285–292.
Ho K, Carman GP 2000 Sputter deposition of NiTi thin film shape memory alloy using a heated target. Thin Solid Films 370: 18–29
Stepan LL, Levi DL, Carman GP 2005 A thin film nitinol heart valve. J Biomech Eng 127: 915–918
Gewillig M, Boshoff DE, Dens J, Mertens L, Benson LN 2004 Stenting the neonatal arterial duct in duct-dependent pulmonary circulation: new techniques, better results. J Am Coll Cardiol 43: 107–112
Ullakko K, Huang JK, Kantner C, Kokorin VV, O'Handley RC 1996 Large magnetic-field-inducted strains in Ni2MnGa signal crystals. Appl Phys Lett 69: 1966–1968
Murray SJ, Marioni M, Allen SM, O'Handley RC, Lograsso TA 2000 6% magnetic-field-induced strain by twin-boundary motion in ferromagnetic Ni-Mn-Ga. Appl Phys Lett 77: 886–888
Sozinov A, Likhachev AA, Lanska N, Ullakko K 2002 Giant magnetic-field-induced strain in NiMnGa seven-layered martenistic phase. Appl Phys Lett 80: 1746–1748
Sozinov A, Likhachev AA, Ullakko K 2002 Crystal structures and magnetic anisotropy properties of Ni-Mn-Ga martensitic phases with giant magnetic-field-induced strain. IEEE Trans Magn 38: 2814–2816
Gans E, Henry D, Carman GP 2004 Reduction in required magnetic field to induce twin-boundary motion in ferromagnetic shape memory alloys. J Appl Phys 95: 6965
Gans E, Carman GP 2006 Cyclic actuation of Ni–Mn–Ga composites. J Appl Phys 99: 084905
Prabhu SD 2007 Altered left ventricular-arterial coupling precedes pump dysfunction in early heart failure. Heart Vessels 22: 170–177
Krishnamoorthy VK, Sengupta PP, Gentile F, Khandheria BK 2007 History of echocardiography and its future applications in medicine. Crit Care Med 35: S309–S313
Hall LT, Maple JL, Agzatian J, Abbot D 2000 Sensor system for heart sound biomonitor. Microelectron J 31: 583–592
Lanning C, Shandas R 2003 Development and validation of implantable sensors for monitoring function of prosthetic heart valves: in vitro studies. Med Biol Eng Comput 41: 416–424
Elle OJ, Halvorsen S, Gulbrandsen MG, Aurdal L, Bakken A, Samset E, Dugstad H, Fosse E 2005 Early recognition of regional cardiac ischemia using a 3-axis accelerometer sensor. Physiol Meas 26: 429–440
Jang LS, Kan WH 2007 Peristaltic piezoelectric micropump system for biomedical applications. Biomed Microdevices 9: 619–626
Doll A, Wischke M, Schrag HJ, Geipel A, Goldschmidtboeing F, Woias P 2007 Characterization of active silicon microvalves with piezoelectric membrane actuators. Microelectron Eng 84: 1202–1206
Geipel A, Doll A, Jantscheff P, Esser N, Massing U, Woias P, Goldschmidtboeing F 2007 A novel two-stage backpressure-independent micropump: modeling and characterization. J Micromech Microeng 17: 949–959
Williams MJ Jr, Welkowitz W, Fich S, Molony DA, Jaron D, Kantrowitz A 1975 The design of a piezoelectric heart assist device. IEEE Trans Biomed Eng 22: 40–46
Siess T, Nix C, Menzler F 2001 From a lab type to a product: a retrospective view on impella's assist technology. Artif Organs 25: 414–421
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Levi, D., Kusnezov, N. & Carman, G. Smart Materials Applications for Pediatric Cardiovascular Devices. Pediatr Res 63, 552–558 (2008). https://doi.org/10.1203/PDR.0b013e31816a9d18
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1203/PDR.0b013e31816a9d18
This article is cited by
-
Application of an arched, Ni–Ti shape-memory connector in repairing distal tibiofibular syndesmosis ligament injury
BMC Musculoskeletal Disorders (2022)
-
Smart advanced responsive materials, synthesis methods and classifications: from Lab to applications
Journal of Polymer Research (2021)
-
A Review on Additive Manufacturing of Shape-Memory Materials for Biomedical Applications
JOM (2020)
-
Evaluation of NaOH pre-treatment on the corrosion behavior and surface characteristics of hydroxyapatite coated NiTi alloy
Applied Physics A (2020)
-
Fabrication and Characterization of Freestanding NiTi Based Thin Film Materials for Shape Memory Micro-actuator Applications
Shape Memory and Superelasticity (2019)