Abstract
Objectives: To determine whether COX-2, bcl-2 and neoangiogenesis are related to human prostate cancer relapse after definitive surgical treatment and progression toward androgen independence and to evaluate the association between the patterns of these tumoral biomarkers and other standard clinico-pathological parameters (such as Gleason score, PSA, TNM stage).
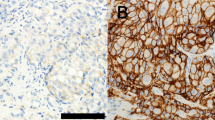
Materials and Methods: We retrospectively analyzed the records on 126 prostate cancer samples from patients treated at our University Hospital from 1995 to 2002. The 72 patients with clinically localized disease (group 1) had undergone radical prostatectomy. Another 54 patients (group 2) had metastatic androgen-independent disease. Archived material relating to the subjects was then immunostained for bcl-2, COX-2 and CD-31, using an anti-bcl-2 monoclonal primary antibody, an anti-COX-2 polyclonal rabbit antibody and an anti-CD-31 monoclonal mouse antibody to evaluate neoangiogenesis (MVD, microvessel density).
Results: We found that bcl-2, COX-2 and MVD expression increased from group 1 to group 2. The intergroup difference was significant only for high MVD (P<0.05). On the other hand, high MVD, high bcl-2 and high COX-2 expression was correlated with a higher PSA level (P<0.01), whereas only a high MVD was also related with Gleason score (P<0.05). We used univariate analysis to evaluate the prognostic impact of biologic and clinico-pathologic parameters on the disease-free-survival of 72 patients treated by radical prostatectomy. A total of 30 patients (41.6%) experienced biochemical relapse; bcl-2, COX-2 and MVD significantly correlated with disease relapse in these patients. In fact, we observed disease relapse in 24/45 (53%) with high bcl-2 expression, in 15/21 (71%) with a high MVD count and finally, in 30/58 (52%) with high COX-2 expression. Finally, PSA value and Gleason score were the only two biologic markers significantly associated to disease relapse in a multivariate analysis.
Conclusions: Our results strongly support a role for bcl-2, COX-2 and angiogenesis in the development and progression of prostate cancer. Of course, we are aware of the small sample size considered in our study. Further investigations would better clarify the prognostic and therapeutic implications of these findings.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 4 print issues and online access
$259.00 per year
only $64.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Walsh PC, Partin AW, Epstein JI . Cancer control and quality of life following anatomical radical retropubic prostatectomy: results at 10 years. J Urol 1994; 152: 1831.
Crawford ED, Rosenblum M, Ziada AM, Lange PH . Overview: hormone refractory prostate cancer. Urology 2000; 54: 1–7.
Autorino R et al. Role of chemotherapy in hormone refractory prostate cancer. Old issues, recent advances and new perspectives. Urol Int 2003; 70: 1–14.
Barton J, Blackledge G, Wakeling A . Growth factors and their receptors: new targets fro prostate cancer therapy. Urology 2001; (Suppl 58): 114.
Steiner MS . Role of peptide growth factors in the prostate: a review. Urology 1993; 42: 99–110.
Porter AC, Vaillancourt RR . Tyrosine kinase receptor-activated signal trasduction pathways which lead to oncogenesis. Oncogene 1998; 17: 1343.
Di Lorenzo G et al. Expression of epidermal growth factor (EGFR) correlates with disease relapse and progression to androgen-independence in human prostate cancer. Clin Cancer Res 2002; 8: 3438–3444.
Tsujimoto Y, Croce CM . Analysis and structure, transcripts and protein products of bcl-2, the gene involved in human follicular lymphoma. Proc Natl Acad Sci USA 1986; 83: 5214–5218.
Reed JC . Bcl-2 and the regulation of programmed cell death. J Cell Biol 1994; 124: 1–6.
Colombel M et al. Detection of the apoptosis-suppressing oncoprotein bcl-2 in hormone refractory human prostate cancer. Am J Pathol 1993; 143: 380–400.
McDonnell TJ et al. Expression of the protooncogene bcl-2 in the prostate and its association with emergence of androgen-independent prostate cancer. Cancer Res 1992; 52: 6940–6944.
Raffo AJ et al. Overexpression of bcl-2 protects prostate cancer cells from apoptosis in vitro and confers resistance to androgen depletion in vivo. Cancer Res 1995; 55: 4438–4445.
Gleave ME et al. Progression to androgen independence is delayed by adjuvant treatment with antisense bcl-2 oligodeoxynucleotides after castration in the LNCaP prostate model. Clin Cancer Res 1999; 5: 2891–2898.
Leung S et al. Synergistic chemosensitization and inhibition of progression to androgen-independence by antisense bcl-2 oligodeoxynucleotide and paclitaxel in the LNCaP prostate tumor model. Int J Cancer 2001; 91: 846–850.
Miyake H, Tolcher A, Gleave ME . Chemosensitization and delayed androgen-independent recurrence of prostate cancer with the use of antisense bcl-2 oligonucleotides. JNCI 2000; 92: 34–41.
Fernandez A et al. Angiogenic potential of prostate carcinoma cells overexpressing bcl-2. JNCI 2001; 93: 208–213.
Liotta LA, Steeg PS, Stevenson WGS . Cancer metastasis and angiogenesis: an imbalance of positive and negative regulation. Cell 1991; 64: 327–336.
Folkman J, Shing Y . Angiogenesis. J Biol Chem 1992; 267: 10931–10934.
Weidner N et al. Tumor angiogenesis correlates with metastasis in invasive prostate carcinoma. Am. J Pathol 1993; 143: 401–409.
Shih SC et al. Molecular profiling of angiogenesis markers. Am J Pathol 2002; 161: 35–41.
Jackson MW, Bentel JM, Tilley WD . Vascular endothelial growth factor (VEGF) expression in prostate cancer and benign prostatic hyperplasia. J Urol 1997; 157: 2323–2328.
Giri D, Ropiquet F, Ittmann M . FGF is an autocrine and paracrine prostatic growth factor expressed by prostatic stromal cells. J Cell Physiol 1999; 180: 53–60.
Smith WL, DeWitt DL, Garavito RM . Cyclooxygenases: structural, cellular and molecular biology. Annu Rev Biochem 2000; 69: 145–182.
Hussain T, Gupta S, Mukhtar H . Cyclooxygenase-2 and prostate carcinogenesis. Cancer Lett 2003; 191: 125–135.
Liu XH et al. Inhibition of cyclooxygenase-2 suppresses angiogenesis and the growth of prostate cancer in vivo. J Urol 2000; 164: 820–825.
Tsujii M, DuBois RN . Alterations in cellular adhesion and apoptosis in epithelial cells overexpressing prostaglandin endoperoxide synthase 2. Cell 1995; 83: 493–501.
Gupta S et al. Overexpression of cyclooxygenase-2 in human prostate adenocarcinoma. Prostate 2000; 42: 73–78.
Kirschenbaum A et al. Expression of cyclooxygenase-1 and cyclooxygenase-2 in the human prostate. Urology 2000; 56: 671–676.
Yoshimura R et al. Expression of cyclooxygenase-2 in prostate carcinoma. Cancer 2000; 89: 589–596.
Zha S et al. Cyclooxygenase-2 is up regulated in proliferative inflammatory atrophy of the prostate, but not in prostate carcinoma. Cancer Res 2001; 61: 8617–8623.
Amling CL et al. Defining prostate specific antigen progression after radical prostatectomy. What is the most appropriate cut point? J Urol 2001; 165: 1146–1151.
Kaplan EL, Meier P . Nonparametric estimation from incomplete observation. J Am Stat Assoc 1958; 53: 457–481.
Mantel N . Evaluation of survival data and two new rank order statistics arising in its consideration. Cancer Chemother Rep 1996; 50: 163–170.
Cox DR . Regression models and life tables. J Roy Statist Soc 1972; 34: 187–220.
Culing Z et al. Expression, structure and function of androgen receptor in advanced prostate cancer. Prostate 1998; 35: 63–70.
Nazareth LV, Weigel NL . Activation of the human androgen receptor through a protein kinase A signaling pathway. J Biol Chem 1996; 271: 19900–19907.
Ye D, Mendelsoh J, Fan Z . Androgen and epidermal growth factor down-regulate cyclin-dependent kinase inhibitor p27 and costimulate proliferation of MDA Pca 2a and MDA PCA 2b prostate cancer cells. Clin Cancer Res 1999; 5: 2171–2177.
Jenster G . Ligand-independent activation of the androgen receptor in prostate cancer by growth factors and cytokines. J Pathol 2000; 191: 227–228.
Catz SD, Johnson JL . Bcl-2 in prostate cancer: a minireview. Apoptosis 2003; 8: 29–37.
Acknowledgements
We thank the excellent technical assistance of Mr Gaetano Borriello, Antonella Verbeni, Ersilia Cerbone. We are grateful to Jean Gilder (Scientific Communication) for editing the text.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Di Lorenzo, G., De Placido, S., Autorino, R. et al. Expression of biomarkers modulating prostate cancer progression: implications in the treatment of the disease. Prostate Cancer Prostatic Dis 8, 54–59 (2005). https://doi.org/10.1038/sj.pcan.4500768
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.pcan.4500768
Keywords
This article is cited by
-
Inhibition of cyclooxygenase-2-mediated matriptase activation contributes to the suppression of prostate cancer cell motility and metastasis
Oncogene (2017)
-
The role of treatment modality on the utility of predictive tissue biomarkers in clinical prostate cancer: a systematic review
Journal of Cancer Research and Clinical Oncology (2013)
-
Lack of association between COX-2 staining level and biochemical recurrence following salvage radiation therapy for recurrent prostate cancer
Journal of Radiation Oncology (2013)
-
p53 and Cyclooxygenase-2 Expression are Directly Associated with Cyclin D1 Expression in Radical Prostatectomy Specimens of Patients with Hormone-Naïve Prostate Cancer
Pathology & Oncology Research (2012)
-
ADAMTS1 alters blood vessel morphology and TSP1 levels in LNCaP and LNCaP-19 prostate tumors
BMC Cancer (2010)