Abstract
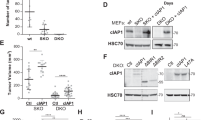
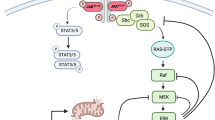
Janus kinase 2 (JAK2) couples ligand activation of cell surface cytokine receptors to the regulation of cellular functions including cell cycle progression, differentiation and apoptosis. It thereby coordinates biological programs such as development and hematopoiesis. Unscheduled activation of JAK2 by point mutations or chromosomal translocations can induce hyperproliferation and hematological malignancies. Typical signal transduction by the JAK2 tyrosine kinase comprises phosphorylation of STAT transcription factors. In this study, we describe the identification of the cyclin-dependent kinase (CDK) inhibitor p27Kip1 as a novel JAK2 substrate. JAK2 can directly bind and phosphorylate p27Kip1. Both, the JAK2 FERM domain and its kinase domain bind to p27Kip1. JAK2 phosphorylates tyrosine residue 88 (Y88) of p27Kip1. We previously reported that Y88 phosphorylation of p27Kip1 by oncogenic tyrosine kinases impairs p27Kip1-mediated CDK inhibition, and initiates its ubiquitin-dependent proteasomal degradation. Consistently, we now find that active oncogenic JAK2V617F reduces p27Kip1 stability and protein levels in patient-derived cell lines harboring the mutant JAK2V617F allele. Moreover, tyrosine phosphorylation of p27Kip1 is impaired and p27Kip1 expression is restored upon JAK2V617F inactivation by small hairpin RNA-mediated knockdown or by the pyridone-containing tetracycle JAK inhibitor-I, indicating that direct phosphorylation of p27Kip1 can contribute to hyperproliferation of JAK2V617F-transformed cells. Activation of endogenous JAK2 by interleukin-3 (IL-3) induces Y88 phosphorylation of p27Kip1, thus unveiling a novel link between cytokine signaling and cell cycle control in non-transformed cells. Oncogenic tyrosine kinases could use this novel pathway to promote hyperproliferation in tumor cells.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Agarwal A, Bumm TG, Corbin AS, O'Hare T, Loriaux M, VanDyke J et al. (2008). Absence of SKP2 expression attenuates BCR-ABL-induced myeloproliferative disease. Blood 112: 1960–1970.
Baker SJ, Rane SG, Reddy EP . (2007). Hematopoietic cytokine receptor signaling. Oncogene 26: 6724–6737.
Behrmann I, Smyczek T, Heinrich PC, Schmitz-Van de Leur H, Komyod W, Giese B et al. (2004). Janus kinase (Jak) subcellular localization revisited: the exclusive membrane localization of endogenous Janus kinase 1 by cytokine receptor interaction uncovers the Jak.receptor complex to be equivalent to a receptor tyrosine kinase. J Biol Chem 279: 35486–35493.
Bruchova H, Yoon D, Agarwal AM, Swierczek S, Prchal JT . (2009). Erythropoiesis in polycythemia vera is hyper-proliferative and has accelerated maturation. Blood Cells Mol Dis 43: 81–87.
Chu I, Sun J, Arnaout A, Kahn H, Hanna W, Narod S et al. (2007). p27 phosphorylation by Src regulates inhibition of cyclin E-Cdk2. Cell 128: 281–294.
Chu IM, Hengst L, Slingerland JM . (2008). The Cdk inhibitor p27 in human cancer: prognostic potential and relevance to anticancer therapy. Nat Rev Cancer 8: 253–267.
Connor MK, Kotchetkov R, Cariou S, Resch A, Lupetti R, Beniston RG et al. (2003). CRM1/Ran-mediated nuclear export of p27(Kip1) involves a nuclear export signal and links p27 export and proteolysis. Mol Biol Cell 14: 201–213.
Dawson MA, Bannister AJ, Gottgens B, Foster SD, Bartke T, Green AR et al. (2009). JAK2 phosphorylates histone H3Y41 and excludes HP1alpha from chromatin. Nature 461: 819–822.
Desrivieres S, Kunz C, Barash I, Vafaizadeh V, Borghouts C, Groner B . (2006). The biological functions of the versatile transcription factors STAT3 and STAT5 and new strategies for their targeted inhibition. J Mammary Gland Biol Neoplasia 11: 75–87.
Feng J, Witthuhn BA, Matsuda T, Kohlhuber F, Kerr IM, Ihle JN . (1997). Activation of Jak2 catalytic activity requires phosphorylation of Y1007 in the kinase activation loop. Mol Cell Biol 17: 2497–2501.
Frescas D, Pagano M . (2008). Deregulated proteolysis by the F-box proteins SKP2 and beta-TrCP: tipping the scales of cancer. Nat Rev Cancer 8: 438–449.
Furuhata A, Kimura A, Shide K, Shimoda K, Murakami M, Ito H et al. (2009). p27 deregulation by Skp2 overexpression induced by the JAK2V617 mutation. Biochem Biophys Res Commun 383: 411–416.
Grimmler M, Wang Y, Mund T, Cilensek Z, Keidel EM, Waddell MB et al. (2007). Cdk-inhibitory activity and stability of p27Kip1 are directly regulated by oncogenic tyrosine kinases. Cell 128: 269–280.
Halfter H, Friedrich M, Resch A, Kullmann M, Stogbauer F, Ringelstein EB et al. (2006). Oncostatin M induces growth arrest by inhibition of Skp2, Cks1, and cyclin A expression and induced p21 expression. Cancer Res 66: 6530–6539.
Hengst L, Dulic V, Slingerland JM, Lees E, Reed SI . (1994). A cell cycle-regulated inhibitor of cyclin-dependent kinases. Proc Natl Acad Sci USA 91: 5291–5295.
Hengst L, Reed SI . (1996). Translational control of p27Kip1 accumulation during the cell cycle. Science 271: 1861–1864.
Ihle JN, Gilliland DG . (2007). Jak2: normal function and role in hematopoietic disorders. Curr Opin Genet Dev 17: 8–14.
James C, Ugo V, Le Couedic JP, Staerk J, Delhommeau F, Lacout C et al. (2005). A unique clonal JAK2 mutation leading to constitutive signalling causes polycythaemia vera. Nature 434: 1144–1148.
James MK, Ray A, Leznova D, Blain SW . (2008). Differential modification of p27Kip1 controls its cyclin D-cdk4 inhibitory activity. Mol Cell Biol 28: 498–510.
Kardinal C, Dangers M, Kardinal A, Koch A, Brandt DT, Tamura T et al. (2006). Tyrosine phosphorylation modulates binding preference to cyclin-dependent kinases and subcellular localization of p27Kip1 in the acute promyelocytic leukemia cell line NB4. Blood 107: 1133–1140.
Kelvin DJ, Chance S, Shreeve M, Axelrad AA, Connolly JA, McLeod D . (1986). Interleukin 3 and cell cycle progression. J Cell Physiol 127: 403–409.
Kralovics R, Passamonti F, Buser AS, Teo SS, Tiedt R, Passweg JR et al. (2005). A gain-of-function mutation of JAK2 in myeloproliferative disorders. N Engl J Med 352: 1779–1790.
LaBaer J, Garrett MD, Stevenson LF, Slingerland JM, Sandhu C, Chou HS et al. (1997). New functional activities for the p21 family of CDK inhibitors. Genes Dev 11: 847–862.
Larrea MD, Liang J, Da Silva T, Hong F, Shao SH, Han K et al. (2008). Phosphorylation of p27Kip1 regulates assembly and activation of cyclin D1-Cdk4. Mol Cell Biol 28: 6462–6472.
Levine RL, Gilliland DG . (2007). JAK-2 mutations and their relevance to myeloproliferative disease. Curr Opin Hematol 14: 43–47.
Levine RL, Wadleigh M, Cools J, Ebert BL, Wernig G, Huntly BJ et al. (2005). Activating mutation in the tyrosine kinase JAK2 in polycythemia vera, essential thrombocythemia, and myeloid metaplasia with myelofibrosis. Cancer Cell 7: 387–397.
Malumbres M, Barbacid M . (2009). Cell cycle, CDKs and cancer: a changing paradigm. Nat Rev Cancer 9: 153–166.
Morgan DO . (1997). Cyclin-dependent kinases: engines, clocks, and microprocessors. Annu Rev Cell Dev Biol 13: 261–291.
Neilson LM, Zhu J, Xie J, Malabarba MG, Sakamoto K, Wagner KU et al. (2007). Coactivation of janus tyrosine kinase (Jak)1 positively modulates prolactin-Jak2 signaling in breast cancer: recruitment of ERK and signal transducer and activator of transcription (Stat)3 and enhancement of Akt and Stat5a/b pathways. Mol Endocrinol 21: 2218–2232.
Parganas E, Wang D, Stravopodis D, Topham DJ, Marine JC, Teglund S et al. (1998). Jak2 is essential for signaling through a variety of cytokine receptors. Cell 93: 385–395.
Ray A, James MK, Larochelle S, Fisher RP, Blain SW . (2009). p27Kip1 inhibits cyclin D-cyclin-dependent kinase 4 by two independent modes. Mol Cell Biol 29: 986–999.
Sarbassov DD, Guertin DA, Ali SM, Sabatini DM . (2005). Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science 307: 1098–1101.
Thompson JE, Cubbon RM, Cummings RT, Wicker LS, Frankshun R, Cunningham BR et al. (2002). Photochemical preparation of a pyridone containing tetracycle: a Jak protein kinase inhibitor. Bioorg Med Chem Lett 12: 1219–1223.
Tossidou I, Dangers M, Koch A, Brandt DT, Schiffer M, Kardinal C . (2008). Tyrosine phosphatase SHP-2 is a regulator of p27(Kip1) tyrosine phosphorylation. Cell Cycle 7: 3858–3868.
Verstovsek S . (2009). Therapeutic potential of JAK2 inhibitors. Hematol Am Soc Hematol Educ Program, 636–642.
Wallace TA, Sayeski PP . (2006). Jak2 tyrosine kinase: a mediator of both housekeeping and ligand-dependent gene expression? Cell Biochem Biophys 44: 213–222.
Walz C, Crowley BJ, Hudon HE, Gramlich JL, Neuberg DS, Podar K et al. (2006). Activated Jak2 with the V617F point mutation promotes G1/S phase transition. J Biol Chem 281: 18177–18183.
Wei W, Ayad NG, Wan Y, Zhang GJ, Kirschner MW, Kaelin Jr WG . (2004). Degradation of the SCF component Skp2 in cell-cycle phase G1 by the anaphase-promoting complex. Nature 428: 194–198.
Wernig G, Gonneville JR, Crowley BJ, Rodrigues MS, Reddy MM, Hudon HE et al. (2008). The Jak2V617F oncogene associated with myeloproliferative diseases requires a functional FERM domain for transformation and for expression of the Myc and Pim proto-oncogenes. Blood 111: 3751–3759.
Zhao R, Xing S, Li Z, Fu X, Li Q, Krantz SB et al. (2005). Identification of an acquired JAK2 mutation in polycythemia vera. J Biol Chem 280: 22788–22792.
Zhao Y, Wagner F, Frank SJ, Kraft AS . (1995). The amino-terminal portion of the JAK2 protein kinase is necessary for binding and phosphorylation of the granulocyte-macrophage colony-stimulating factor receptor beta c chain. J Biol Chem 270: 13814–13818.
Acknowledgements
We thank Jakob Troppmair and Justus Duyster for providing cell lines, Wolfgang Doppler for providing plasmids and antibodies and Stephan Geley for providing plasmids. We thank Wolfgang Doppler, Michael Kullmann, Jonathan Vosper and all members of the Hengst lab for support, stimulating discussions and critical reading of the manuscript. The work was funded by the FWF Grants SFB F21-B12 and P18873-B1.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on the Oncogene website
Supplementary information
Rights and permissions
About this article
Cite this article
Jäkel, H., Weinl, C. & Hengst, L. Phosphorylation of p27Kip1 by JAK2 directly links cytokine receptor signaling to cell cycle control. Oncogene 30, 3502–3512 (2011). https://doi.org/10.1038/onc.2011.68
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2011.68
Keywords
This article is cited by
-
Inability to phosphorylate Y88 of p27Kip1 enforces reduced p27 protein levels and accelerates leukemia progression
Leukemia (2022)
-
ACK1 upregulated the proliferation of head and neck squamous cell carcinoma cells by promoting p27 phosphorylation and degradation
Journal of Cell Communication and Signaling (2022)
-
The CDK inhibitor p57Kip2 enhances the activity of the transcriptional coactivator FHL2
Scientific Reports (2020)
-
Phosphoserine aminotransferase 1 is associated to poor outcome on tamoxifen therapy in recurrent breast cancer
Scientific Reports (2017)
-
Suppressor of Cytokine Signaling 2 Negatively Regulates NK Cell Differentiation by Inhibiting JAK2 Activity
Scientific Reports (2017)