Abstract
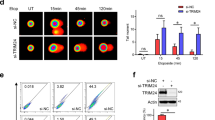
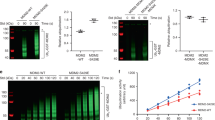
The p53 tumour-suppressor protein is tightly regulated through its association with the Hdm2 E3 ligase. Activation of p53 by DNA strand breaks is orchestrated by the ataxia-telangiectasia mutated (ATM) protein kinase and involves interruption of Hdm2-mediated p53 degradation. As part of this mechanism ATM itself, and the ATM-activated protein tyrosine kinase, c-Abl, inhibit Hdm2 function through phosphorylation of serine 395 and tyrosine 394 (Y394), respectively. In the present study, we have identified a novel target of c-Abl in the Hdm2 protein, tyrosine 276 (Y276). We show that c-Abl phosphorylates this residue in vitro and confirm that Y394 is a target of c-Abl. We also show that Y276 is phosphorylated in a c-Abl-dependent manner in cultured cells and provide evidence that Y276 is phosphorylated in response to DNA damage coincident with the activation of c-Abl. Finally, we show that Y276 phosphorylation stimulates interaction with ARF, leading to increased levels of nucleolar Hdm2 and decreased turnover of p53. These data establish Y276 as a physiological target of c-Abl that contributes functionally to the induction of p53.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Appella E, Anderson CW . (2001). Eur J Biochem 268: 2764–2772.
Barila D, Mangano R, Gonfloni S, Kretzschmar J, Moro M, Bohmann D et al. (2000). EMBO J 19: 273–281.
Blattner C, Hay TJ, Meek DW, Lane DP . (2002). Mol Cell Biol 22: 6170–6182.
Bothner B, Lewis WS, DiGiammarino EL, Weber JD, Bothner SJ, Kriwacki RW . (2001). J Mol Biol 314: 263–277.
Goldberg Z, Sionov RV, Berger M, Zwang Y, Perets R, Van Etten RA et al. (2002). EMBO J 21: 3715–3727.
Harris SL, Levine AJ . (2005). Oncogene 24: 2899–2908.
Kurki S, Peltonen K, Kiviharju T, Latonen L, Ojala P, Meek D et al. (2004). Cancer Cell 5: 465–475.
Lu X . (2005). Curr Opin Genet Dev 15: 27–33.
Maya R, Balass M, Kim ST, Shkedy D, Leal JF, Shifman O et al. (2001). Genes Dev 15: 1067–1077.
Meek DW, Knippschild U . (2003). Mol Cancer Res 1: 1017–1026.
Michael D, Oren M . (2003). Semin Cancer Biol 13: 49–58.
Saito S, Yamaguchi H, Higashimoto Y, Chao C, Xu Y, Fornace Jr AJ et al. (2003). J Biol Chem 278: 37536–37544.
Shaul Y, Ben-Yehoyada M . (2005). Cell Res 15: 33–35.
Sionov RV, Coen S, Goldberg Z, Berger M, Bercovich B, Ben-Neriah Y et al. (2001). Mol Cell Biol 21: 5869–5878.
Sionov RV, Moallem E, Berger M, Kazaz A, Gerlitz O, Ben-Neriah Y et al. (1999). J Biol Chem 274: 8371–8374.
Wu JJ, Afar DE, Phan H, Witte ON, Lam KS . (2002). Comb Chem High Throughput Screen 5: 83–91.
Acknowledgements
We are extremely grateful to Giulio Superti-Furga for the gift of plasmids expressing the c-Abl kinase, to Sonia Lain for ARF-expressing plasmid and antibody to Mark Saville for purified Hdm2 and to Frances Fuller-Pace for assistance with immunofluorescence microscopy. This work was supported by the Biomedical Research Centre (University of Dundee), the Association for International Cancer Research and Cancer Research UK.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Dias, S., Milne, D. & Meek, D. c-Abl phosphorylates Hdm2 at tyrosine 276 in response to DNA damage and regulates interaction with ARF. Oncogene 25, 6666–6671 (2006). https://doi.org/10.1038/sj.onc.1209671
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.onc.1209671
Keywords
This article is cited by
-
LAMP3 plays an oncogenic role in osteosarcoma cells partially by inhibiting TP53
Cellular & Molecular Biology Letters (2018)
-
Mdm2 links genotoxic stress and metabolism to p53
Protein & Cell (2010)
-
ARF promotes accumulation of retinoblastoma protein through inhibition of MDM2
Oncogene (2007)