Abstract
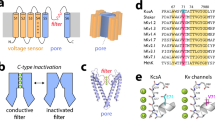
C-type inactivation underlies important roles played by voltage-gated K+ (Kv) channels. Functional studies have provided strong evidence that a common underlying cause of this type of inactivation is an alteration near the extracellular end of the channel's ion-selectivity filter. Unlike N-type inactivation, which is known to reflect occlusion of the channel's intracellular end, the structural mechanism of C-type inactivation remains controversial and may have many detailed variations. Here we report that in voltage-gated Shaker K+ channels lacking N-type inactivation, a mutation enhancing inactivation disrupts the outermost K+ site in the selectivity filter. Furthermore, in a crystal structure of the Kv1.2-2.1 chimeric channel bearing the same mutation, the outermost K+ site, which is formed by eight carbonyl-oxygen atoms, appears to be slightly too small to readily accommodate a K+ ion and in fact exhibits little ion density; this structural finding is consistent with the functional hallmark of C-type inactivation.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Hodgkin, A.L. & Huxley, A.F. A quantitative description of membrane current and its application to conduction and excitation in nerve. J. Physiol. (Lond.) 117, 500–544 (1952).
Armstrong, C.M. Interaction of tetraethylammonium ion derivatives with the potassium channels of giant axons. J. Gen. Physiol. 58, 413–437 (1971).
Hoshi, T., Zagotta, W.N. & Aldrich, R.W. Biophysical and molecular mechanisms of Shaker potassium channel inactivation. Science 250, 533–538 (1990).
Zagotta, W.N., Hoshi, T. & Aldrich, R.W. Restoration of inactivation in mutants of Shaker potassium channels by a peptide derived from ShB. Science 250, 568–571 (1990).
Hoshi, T., Zagotta, W.N. & Aldrich, R.W. Two types of inactivation in Shaker K+ channels: effects of alterations in the carboxy-terminal region. Neuron 7, 547–556 (1991).
Rettig, J. et al. Inactivation properties of voltage-gated K+ channels altered by presence of β-subunit. Nature 369, 289–294 (1994).
Choi, K.L., Mossman, C., Aubé, J. & Yellen, G. The internal quaternary ammonium receptor site of Shaker potassium channels. Neuron 10, 533–541 (1993).
Liu, Y., Holmgren, M., Jurman, M.E. & Yellen, G. Gated access to the pore of a voltage-dependent K+ channel. Neuron 19, 175–184 (1997).
Doyle, D.A. et al. The structure of the potassium channel: molecular basis of K+ conduction and selectivity. Science 280, 69–77 (1998).
López-Barneo, J., Hoshi, T., Heinemann, S.H. & Aldrich, R.W. Effects of external cations and mutations in the pore region on C-type inactivation of Shaker potassium channels. Receptors Channels 1, 61–71 (1993).
Yang, Y., Yan, Y. & Sigworth, F.J. How does the W434F mutation block current in Shaker potassium channels? J. Gen. Physiol. 109, 779–789 (1997).
Yellen, G., Sodickson, D., Chen, T.Y. & Jurman, M.E. An engineered cysteine in the external mouth of a K+ channel allows inactivation to be modulated by metal binding. Biophys. J. 66, 1068–1075 (1994).
Long, S.B., Campbell, E.B. & Mackinnon, R. Crystal structure of a mammalian voltage-dependent Shaker family K+ channel. Science 309, 897–903 (2005).
Long, S.B., Tao, X., Campbell, E.B. & MacKinnon, R. Atomic structure of a voltage-dependent K+ channel in a lipid membrane-like environment. Nature 450, 376–382 (2007).
Hoshi, T. & Armstrong, C.M. C-type inactivation of voltage-gated K+ channels: pore constriction or dilation? J. Gen. Physiol. 141, 151–160 (2013).
Armstrong, C.M. & Hoshi, T. K+ channel gating: C-type inactivation is enhanced by calcium or lanthanum outside. J. Gen. Physiol. 144, 221–230 (2014).
Monod, J., Wyman, J. & Changeux, J.P. On the nature of allosteric transitions: a plausible model. J. Mol. Biol. 12, 88–118 (1965).
Perozo, E., MacKinnon, R., Bezanilla, F. & Stefani, E. Gating currents from a nonconducting mutant reveal open-closed conformations in Shaker K+ channels. Neuron 11, 353–358 (1993).
Hackos, D.H., Chang, T.H. & Swartz, K.J. Scanning the intracellular S6 activation gate in the shaker K+ channel. J. Gen. Physiol. 119, 521–532 (2002).
Kitaguchi, T., Sukhareva, M. & Swartz, K.J. Stabilizing the closed S6 gate in the Shaker Kv channel through modification of a hydrophobic seal. J. Gen. Physiol. 124, 319–332 (2004).
Armstrong, C.M. & Bezanilla, F. Currents related to movement of the gating particles of the sodium channels. Nature 242, 459–461 (1973).
Garcia, M.L., Garcia-Calvo, M., Hidalgo, P., Lee, A. & MacKinnon, R. Purification and characterization of three inhibitors of voltage-dependent K+ channels from Leiurus quinquestriatus var. hebraeus venom. Biochemistry 33, 6834–6839 (1994).
MacKinnon, R. & Miller, C. Mechanism of charybdotoxin block of the high-conductance, Ca2+-activated K+ channel. J. Gen. Physiol. 91, 335–349 (1988).
Park, C.S. & Miller, C. Interaction of charybdotoxin with permeant ions inside the pore of a K+ channel. Neuron 9, 307–313 (1992).
Hidalgo, P. & MacKinnon, R. Revealing the architecture of a K+ channel pore through mutant cycles with a peptide inhibitor. Science 268, 307–310 (1995).
Banerjee, A., Lee, A., Campbell, E. & Mackinnon, R. Structure of a pore-blocking toxin in complex with a eukaryotic voltage-dependent K+ channel. eLife 2, e00594 (2013).
Tao, X., Lee, A., Limapichat, W., Dougherty, D.A. & MacKinnon, R. A gating charge transfer center in voltage sensors. Science 328, 67–73 (2010).
Webster, S.M., Del Camino, D., Dekker, J.P. & Yellen, G. Intracellular gate opening in Shaker K+ channels defined by high-affinity metal bridges. Nature 428, 864–868 (2004).
Heginbotham, L., Lu, Z., Abramson, T. & MacKinnon, R. Mutations in the K+ channel signature sequence. Biophys. J. 66, 1061–1067 (1994).
Baukrowitz, T. & Yellen, G. Modulation of K+ current by frequency and external [K+]: a tale of two inactivation mechanisms. Neuron 15, 951–960 (1995).
Baukrowitz, T. & Yellen, G. Use-dependent blockers and exit rate of the last ion from the multi-ion pore of a K+ channel. Science 271, 653–656 (1996).
Harris, R.E., Larsson, H.P. & Isacoff, E.Y. A permanent ion binding site located between two gates of the Shaker K+ channel. Biophys. J. 74, 1808–1820 (1998).
Panyi, G. & Deutsch, C. Cross talk between activation and slow inactivation gates of Shaker potassium channels. J. Gen. Physiol. 128, 547–559 (2006).
Zhou, Y., Morais-Cabral, J.H., Kaufman, A. & MacKinnon, R. Chemistry of ion coordination and hydration revealed by a K+ channel–Fab complex at 2.0 A resolution. Nature 414, 43–48 (2001).
Cordero-Morales, J.F. et al. Molecular determinants of gating at the potassium-channel selectivity filter. Nat. Struct. Mol. Biol. 13, 311–318 (2006).
Cuello, L.G., Jogini, V., Cortes, D.M. & Perozo, E. Structural mechanism of C-type inactivation in K+ channels. Nature 466, 203–208 (2010).
Matulef, K., Komarov, A.G., Costantino, C.A. & Valiyaveetil, F.I. Using protein backbone mutagenesis to dissect the link between ion occupancy and C-type inactivation in K+ channels. Proc. Natl. Acad. Sci. USA 110, 17886–17891 (2013).
Matulef, K., Annen, A.W., Nix, J.C. & Valiyaveetil, F.I. Individual ion binding sites in the K+ channel play distinct roles in C-type inactivation and in recovery from inactivation. Structure 24, 750–761 (2016).
Trudeau, M.C., Warmke, J.W., Ganetzky, B. & Robertson, G.A. HERG, a human inward rectifier in the voltage-gated potassium channel family. Science 269, 92–95 (1995).
Smith, P.L., Baukrowitz, T. & Yellen, G. The inward rectification mechanism of the HERG cardiac potassium channel. Nature 379, 833–836 (1996).
Wang, W. & MacKinnon, R. Cryo-EM structure of the open human Ether-à-go-go-related K+ channel hERG. Cell 169, 422–430.e10 (2017).
Schönherr, R. & Heinemann, S.H. Molecular determinants for activation and inactivation of HERG, a human inward rectifier potassium channel. J. Physiol. (Lond.) 493, 635–642 (1996).
Liman, E.R., Tytgat, J. & Hess, P. Subunit stoichiometry of a mammalian K+ channel determined by construction of multimeric cDNAs. Neuron 9, 861–871 (1992).
McCormack, K., Lin, L., Iverson, L.E., Tanouye, M.A. & Sigworth, F.J. Tandem linkage of Shaker K+ channel subunits does not ensure the stoichiometry of expressed channels. Biophys. J. 63, 1406–1411 (1992).
Otwinowski, Z. & Minor, W. Processing of X-ray diffraction data collected in oscillation mode. Methods Enzymol. 276, 307–326 (1997).
Collaborative Computational Project, Number 4. The CCP4 suite: programs for protein crystallography. Acta Crystallogr. D Biol. Crystallogr. 50, 760–763 (1994).
Brünger, A.T. et al. Crystallography & NMR system: a new software suite for macromolecular structure determination. Acta Crystallogr. D Biol. Crystallogr. 54, 905–921 (1998).
Emsley, P. & Cowtan, K. Coot: model-building tools for molecular graphics. Acta Crystallogr. D Biol. Crystallogr. 60, 2126–2132 (2004).
DeLano, W.L. The PyMOL Molecular Graphics System (DeLano Scientific, 2002).
Adams, P.D. et al. PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr. D Biol. Crystallogr. 66, 213–221 (2010).
Spassova, M. & Lu, Z. Coupled ion movement underlies rectification in an inward-rectifier K+ channel. J. Gen. Physiol. 112, 211–221 (1998).
Acknowledgements
We thank P. De Weer and T. Hoshi (University of Pennsylvania) for critical review of our manuscript; S. Stayrook (University of Pennsylvania) for technical assistance with the in-house X-ray source; R. MacKinnon (Rockefeller University) for cDNA of the rat Kv1.2 α subunit in the pPIZC-C plasmid and C. Deutsch (University of Pennsylvania) for cDNA of the rat Kv β2 subunit in the PCR3A+ plasmid; S. Long (Memorial Sloan Kettering Cancer Center) for information on Kv expression and cryo-milling; the staff at beamlines 8.2.1 and 8.2.2, ALS, Lawrence Berkeley National Laboratory, and at beamline 23-IDB, APS, Argonne National Laboratory, for their support in data collection. Research reported in this publication was supported by the National Institute of Diabetes and Digestive and Kidney Diseases (DK109919) and the National Institute of General Medical Sciences (GM055560) of the National Institutes of Health to Z.L.
Author information
Authors and Affiliations
Contributions
All authors designed research, performed experiments and analyzed data. V.P., Y.Z., Y.R. and Z.L. wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Integrated supplementary information
Supplementary Figure 1 Comparison of the tetrameric Shaker channel and a concatemer construct.
(a, b) Currents of tetrameric (a) and concatemer (b) channels, recorded from oocytes in the presence of 100 mM extracellular K+, as membrane voltage was stepped from the -80 mV holding potential to 80 mV in 10 mV increments and back to -80 mV. The dashed line identifies the zero current level. (c) Normalized tail currents (mean ± s.e.m.; n = 10 - 12) of tetrameric (open circles) and concatemer (open squares) channels plotted against membrane voltage; curves are fits of Boltzmann functions with midpoint voltage V1/2 (mean ± s.e.m.) of -30 ± 0.5 mV and apparent valence Z of 3.5 ± 0.3 for tetrameric channels or V1/2 of -30 ± 0.6 mV and apparent valence Z of 3.3 ± 0.2 for concatemer channels.
Supplementary Figure 2 Ionic currents of wild-type and mutant Kv1.2 channels, recorded from oocytes in the presence of 100 mM extracellular K+.
Ionic currents of wild-type (a) and mutant Kv1.2 (b) channels, The mutation in Kv1.2 corresponds to V478W in the Shaker channel. Currents were elicited by stepping membrane voltage from the -80 mV holding potential to 80 mV in 10 mV increments and back to -80 mV. The dashed line identifies the zero current level.
Supplementary Figure 3 Illustration of hydrogen bonds between the selectivity filter and the pore helix of Kv1.2-2.1, as defined by distances between 2.5 and 3.3 Å.
Carbon atoms of wild-type selectivity filter of two contiguous subunits (PDB: 2R9R) are shown as green and yellow sticks, oxygen atoms in red, and nitrogen atoms in blue; K+ ions and water molecules are shown as green and magenta spheres, respectively. Hydrogen bonds in the extracellular and intracellular regions are indicated by dashed blue and magenta lines, respectively.
Supplementary Figure 4 Comparison of Val377 positions in wild-type and V406W mutant I structures.
Section of super-positioned wild-type (orange) and mutant (blue) structures around the central axis of the selectivity filter, as viewed from the outside of the cell. The part of electron density (2Fo-Fc composite-omit map contoured at 1.5 σ) corresponding to Val 377 is shown.
Supplementary Figure 5 Comparison of Tyr373 in wild-type Kv1.2-2.1 and mutant I structures.
Structures of Gly372-Tyr373-Gly374 in wild-type (orange) and mutant (blue) channels are shown in two views (a and c versus b and d). The two structures were aligned according to Cα atoms of residues 364-372. The super-positioned Fo-Fc omit map of mutant molecule I, contoured at 5 σ (a, b) and 8 σ (c, d), was calculated with a model of the mutant molecule where Gly372-Tyr373-Gly374 were omitted.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–5 and Supplementary Table 1. (PDF 980 kb)
Rights and permissions
About this article
Cite this article
Pau, V., Zhou, Y., Ramu, Y. et al. Crystal structure of an inactivated mutant mammalian voltage-gated K+ channel. Nat Struct Mol Biol 24, 857–865 (2017). https://doi.org/10.1038/nsmb.3457
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nsmb.3457
This article is cited by
-
Structures of the T cell potassium channel Kv1.3 with immunoglobulin modulators
Nature Communications (2022)
-
A distinct mechanism of C-type inactivation in the Kv-like KcsA mutant E71V
Nature Communications (2022)
-
Structural Basis for pH-gating of the K+ channel TWIK1 at the selectivity filter
Nature Communications (2022)
-
Conformational equilibrium shift underlies altered K+ channel gating as revealed by NMR
Nature Communications (2020)
-
Structural basis for pH gating of the two-pore domain K+ channel TASK2
Nature (2020)