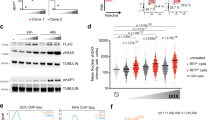
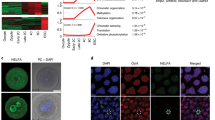
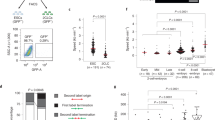
Abstract
Cellular plasticity is essential for early embryonic cells. Unlike pluripotent cells, which form embryonic tissues, totipotent cells can generate a complete organism including embryonic and extraembryonic tissues. Cells resembling 2-cell-stage embryos (2C-like cells) arise at very low frequency in embryonic stem (ES) cell cultures. Although induced reprogramming to pluripotency is well established, totipotent cells remain poorly characterized, and whether reprogramming to totipotency is possible is unknown. We show that mouse 2C-like cells can be induced in vitro through downregulation of the chromatin-assembly activity of CAF-1. Endogenous retroviruses and genes specific to 2-cell embryos are the highest-upregulated genes upon CAF-1 knockdown. Emerging 2C-like cells exhibit molecular characteristics of 2-cell embryos and higher reprogrammability than ES cells upon nuclear transfer. Our results suggest that early embryonic-like cells can be induced by modulating chromatin assembly and that atypical histone deposition may trigger the emergence of totipotent cells.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Accession codes
References
Macfarlan, T.S. et al. Embryonic stem cell potency fluctuates with endogenous retrovirus activity. Nature 487, 57–63 (2012).
Takahashi, K. & Yamanaka, S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 126, 663–676 (2006).
Tarkowski, A.K. Experiments on the development of isolated blastomers of mouse eggs. Nature 184, 1286–1287 (1959).
Ishiuchi, T. & Torres-Padilla, M.E. Towards an understanding of the regulatory mechanisms of totipotency. Curr. Opin. Genet. Dev. 23, 512–518 (2013).
Cahan, P. & Daley, G.Q. Origins and implications of pluripotent stem cell variability and heterogeneity. Nat. Rev. Mol. Cell Biol. 14, 357–368 (2013).
Probst, A.V., Santos, F., Reik, W., Almouzni, G. & Dean, W. Structural differences in centromeric heterochromatin are spatially reconciled on fertilisation in the mouse zygote. Chromosoma 116, 403–415 (2007).
Probst, A.V. et al. A strand-specific burst in transcription of pericentric satellites is required for chromocenter formation and early mouse development. Dev. Cell 19, 625–638 (2010).
Puschendorf, M. et al. PRC1 and Suv39h specify parental asymmetry at constitutive heterochromatin in early mouse embryos. Nat. Genet. 40, 411–420 (2008).
Santenard, A. et al. Heterochromatin formation in the mouse embryo requires critical residues of the histone variant H3.3. Nat. Cell Biol. 12, 853–862 (2010).
Smith, S. & Stillman, B. Purification and characterization of CAF-I, a human cell factor required for chromatin assembly during DNA replication in vitro. Cell 58, 15–25 (1989).
Verreault, A., Kaufman, P.D., Kobayashi, R. & Stillman, B. Nucleosome assembly by a complex of CAF-1 and acetylated histones H3/H4. Cell 87, 95–104 (1996).
Houlard, M. et al. CAF-1 is essential for heterochromatin organization in pluripotent embryonic cells. PLoS Genet. 2, e181 (2006).
Huang, H. et al. Drosophila CAF-1 regulates HP1-mediated epigenetic silencing and pericentric heterochromatin stability. J. Cell Sci. 123, 2853–2861 (2010).
Peaston, A.E. et al. Retrotransposons regulate host genes in mouse oocytes and preimplantation embryos. Dev. Cell 7, 597–606 (2004).
Miyanari, Y., Ziegler-Birling, C. & Torres-Padilla, M.E. Live visualization of chromatin dynamics with fluorescent TALEs. Nat. Struct. Mol. Biol. 20, 1321–1324 (2013).
Rolef Ben-Shahar, T. et al. Two fundamentally distinct PCNA interaction peptides contribute to chromatin assembly factor 1 function. Mol. Cell. Biol. 29, 6353–6365 (2009).
Murzina, N., Verreault, A., Laue, E. & Stillman, B. Heterochromatin dynamics in mouse cells: interaction between chromatin assembly factor 1 and HP1 proteins. Mol. Cell 4, 529–540 (1999).
Kaufman, P.D., Kobayashi, R., Kessler, N. & Stillman, B. The p150 and p60 subunits of chromatin assembly factor I: a molecular link between newly synthesized histones and DNA replication. Cell 81, 1105–1114 (1995).
Nabatiyan, A. & Krude, T. Silencing of chromatin assembly factor 1 in human cells leads to cell death and loss of chromatin assembly during DNA synthesis. Mol. Cell. Biol. 24, 2853–2862 (2004).
Evsikov, A.V. et al. Systems biology of the 2-cell mouse embryo. Cytogenet. Genome Res. 105, 240–250 (2004).
Deng, Q., Ramskold, D., Reinius, B. & Sandberg, R. Single-cell RNA-seq reveals dynamic, random monoallelic gene expression in mammalian cells. Science 343, 193–196 (2014).
Boškovic´, A. et al. Higher chromatin mobility supports totipotency and precedes pluripotency in vivo. Genes Dev. 28, 1042–1047 (2014).
McGrath, J. & Solter, D. Inability of mouse blastomere nuclei transferred to enucleated zygotes to support development in vitro. Science 226, 1317–1319 (1984).
Matoba, S. et al. Embryonic development following somatic cell nuclear transfer impeded by persisting histone methylation. Cell 159, 884–895 (2014).
Smith, S. & Stillman, B. Stepwise assembly of chromatin during DNA replication in vitro. EMBO J. 10, 971–980 (1991).
Takami, Y., Ono, T., Fukagawa, T., Shibahara, K. & Nakayama, T. Essential role of chromatin assembly factor-1-mediated rapid nucleosome assembly for DNA replication and cell division in vertebrate cells. Mol. Biol. Cell 18, 129–141 (2007).
Beddington, R.S. & Robertson, E.J. An assessment of the developmental potential of embryonic stem cells in the midgestation mouse embryo. Development 105, 733–737 (1989).
Morgani, S.M. et al. Totipotent embryonic stem cells arise in ground-state culture conditions. Cell Reports 3, 1945–1957 (2013).
Wood, S.A. et al. Simple and efficient production of embryonic stem cell-embryo chimeras by coculture. Proc. Natl. Acad. Sci. USA 90, 4582–4585 (1993).
Abad, M. et al. Reprogramming in vivo produces teratomas and iPS cells with totipotency features. Nature 502, 340–345 (2013).
Hiiragi, T. & Solter, D. Reprogramming is essential in nuclear transfer. Mol. Reprod. Dev. 70, 417–421 (2005).
Howlett, S.K., Barton, S.C. & Surani, M.A. Nuclear cytoplasmic interactions following nuclear transplantation in mouse embryos. Development 101, 915–923 (1987).
Casanova, M. et al. Heterochromatin reorganization during early mouse development requires a single-stranded noncoding transcript. Cell Reports 4, 1156–1167 (2013).
Miyanari, Y. & Torres-Padilla, M.E. Control of ground-state pluripotency by allelic regulation of Nanog. Nature 483, 470–473 (2012).
Quivy, J.P., Gerard, A., Cook, A.J., Roche, D. & Almouzni, G. The HP1-p150/CAF-1 interaction is required for pericentric heterochromatin replication and S-phase progression in mouse cells. Nat. Struct. Mol. Biol. 15, 972–979 (2008).
Quivy, J.P. et al. A CAF-1 dependent pool of HP1 during heterochromatin duplication. EMBO J. 23, 3516–3526 (2004).
Terranova, R., Sauer, S., Merkenschlager, M. & Fisher, A.G. The reorganisation of constitutive heterochromatin in differentiating muscle requires HDAC activity. Exp. Cell Res. 310, 344–356 (2005).
Maksakova, I.A. et al. H3K9me3-binding proteins are dispensable for SETDB1/H3K9me3-dependent retroviral silencing. Epigenetics Chromatin 4, 12 (2011).
Macfarlan, T.S. et al. Endogenous retroviruses and neighboring genes are coordinately repressed by LSD1/KDM1A. Genes Dev. 25, 594–607 (2011).
Inoue, A., Matoba, S. & Zhang, Y. Transcriptional activation of transposable elements in mouse zygotes is independent of Tet3-mediated 5-methylcytosine oxidation. Cell Res. 22, 1640–1649 (2012).
Kim, D. et al. TopHat2: accurate alignment of transcriptomes in the presence of insertions, deletions and gene fusions. Genome Biol. 14, R36 (2013).
Love, M.I., Huber, W. & Anders, S. Moderated estimation of fold change and dispersion for RNA-Seq data with DESeq2. Genome Biol. 15, 550 (2014).
Anders, S., Pyl, P.T. & Huber, W. HTSeq: a Python framework to work with high-throughput sequencing data. Bioinformatics 31, 166–169 (2015).
Acknowledgements
We thank G. Almouzni and J.P. Quivy (Institut Curie) for providing antibodies to p150 and p60 and for helpful discussions, M. Takeichi (RIKEN Center for Developmental Biology) for pCAG-IRES-Hygro vector, S. Matoba for sharing the list of RRRs, M. Oginuma for help with embryo analysis, B. Jost, M. Philips and S. Vicaire from the Sequencing facility of the Institut Génétique Biologie Moléculaire Cellulaire, C. Ebel for support with FACS, M. Koch for advice on imaging, C. Noll for help in western blotting analysis, R. Diaz-Uriarte for statistical advice, C. Hug for help with code testing, P. André and M. Wattenhofer-Donze from the Institut Clinique de la Souris (ICS) for help with ES-cell work and A.J. Bannister for critical reading of the manuscript. M.-E.T.-P. acknowledges funding from EpiGeneSys NoE, ERC-Stg 'NuclearPotency', the FP7 Marie-Curie Actions ITN Nucleosome4D, the European Molecular Biology Organization Young Investigator Programme and the Fondation Schlumberger pour l'Education et la Recherche. J.M.V. acknowledges funding from EpiGeneSys NoE, Deutsche Forschungsgemeinschaft Cells-in-Motion Cluster of Excellence (EXC 1003–CiM), University of Münster and the Max Planck Society. Work in T.W.'s laboratory is funded through KAKENHI 23248048 and the Takeda Science Foundation. T.I. is supported as a recipient of postdoctoral fellowships from Uehara Memorial Foundation and Human Frontier Science Programme, and A.B. was supported as a recipient of a fellowship from the Association pour la Recherche Contre le Cancer. D.R.-T. is supported by a Dirección General de Cooperación e Internacionalización fellowship from the National University of Mexico. R.E.-G. is a member of the graduate school International Max Planck Research School–Molecular Biomedicine, Münster, Germany. This work received partial support from ANR-10-LABX-0030-INRT.
Author information
Authors and Affiliations
Contributions
T.I. designed and performed most experiments and analyzed data. A.B. performed FRAP, and C.Z.-B. performed Southern blotting. R.E.-G., D.R.-T. and J.M.V. performed computational analyses. E.M. and T.W. performed nuclear transfer. M.-E.T.-P. and J.M.V. analyzed data and directed the study. M.-E.T.-P. wrote the manuscript with input from T.I., R.E.-G. and J.M.V.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Integrated supplementary information
Supplementary Figure 1 Global histone acetylation in 2C-like cells and RNA-FISH for endogenous MERVL and major satellites after p150 depletion.
a. 2C::EGFP ES cells were immunostained for GFP and pan-acetylated H4, H4K16ac,H4K12ac, H4K5/12ac, H4K8/12ac or H3K9ac as indicated. The antibody for pan-acetylated H4 recognizes acetylated H4 K5, K8, K12 or K16. More than 50 cells were analysed in 3 biological replicates. Scale bar, 10 μm.
b. RNA-FISH for MERVL and MajSat was performed in wild type E14 ES cells after transfection of siRNA for control or p150. Scale bar, 50 μm.
Supplementary Figure 2 Effect of p60 KD, Zscan4 activation by CAF-1 KD and use of targeted TALEs to activate major satellite transcription.
a. ES cells transfected with control siRNA or p60 siRNA were immunostained for p60. Scale bar, 10 μm. Note the absence of chromocentrer after p60 loss as revealed by DAPI staining.
b. As in a, but showing several 2C::EGFP ES cell colonies at a lower magnification after p60 RNAi using two different siRNAs.
c. Growth curves of control or p150- or p60-depleted ES cells. Cell growth was analyzed after transfection of siRNA for control, p150 or p60 at the indicated days after transfection. The same number of cells was plated for each condition and cell numbers were counted in parallel. Shown are the mean ± s.d. of two biological replicates.
d. RT-qPCR analysis for Zscan4 expression after control, p150 or p60 RNAi in ES cells. **p<0.01 (Student’s t-test, compared to si control).
e. 2C::EGFP ES cells transfected with control, p150 or p60 siRNA as above and were immunostained for GFP and Zscan4. Scale bar, 100 μm.
f. Upregulation of ZSCAN4 protein levels was assessed by western blot after the transfection of control, p150 or p60 siRNA in ES cells. GAPDH was used as a loading control.
g. 2C::EGFP ES cells were transfected with TALE-Maj-Flag or TALE-Maj-VP64 or mock-transfected and subsequently processed for immunostaining using an antibody for GFP and Flag. Scale bar, 100 μm.
Supplementary Figure 3 G1-S cell-cycle arrest prevents the emergence of 2C-like cells after p150 RNAi and complementation assay for p150.
a. The cell cycle distribution of 2C::EGFP ES cells was analyzed by propidium iodide staining after 24 h treatment of water control or 5mM thymidine.
b. 2C::EGFP ES cells stably expressing full-length p150 or p150 mutants, as indicated on the left of each row, were immunostained for Flag and GFP after the transfection of siRNA control, si p150 #1 or si p150#2. Note that while sip150#2 targets both the endogenous p150 and the exogenous mRNAs, sip150#1 targets exclusively the endogenous p150. Scale bar, 100 μm.
Supplementary Figure 4 RNAi for HIRA in ES cells does not elicit a chromocenter defect or a dramatic upregulation of the 2C::EGFP reporter.
a. Knockdown efficiency for HIRA in 2C::EGFP ES cells was confirmed by western blot. Two different siRNAs were used. GAPDH was used as a loading control.
b. 2C::EGFP ES cells transfected with control siRNA or two different siRNA for HIRA were immunostained for GFP and HIRA. Scale bar, 100 μm. Shown are representative images of two independent biological replicates.
c. Quantification of the percentage of EGFP-positive 2C-like cells by FACS following transfection of siRNA control or two different siRNA for HIRA. Shown are the mean ± s.e.m. of two independent biological replicates. The numbers above the columns indicate the fold change of EGFP-positive cells compared to si control.
d. ES cells transfected with control or HIRA siRNA were immunostained for HIRA. The DNA was stained with DAPI. Scale bar, 10 μm.
Supplementary Figure 5 Analysis of differentially expressed genes and repeats after RNAi for CAF-1 subunits p150 or p60.
a. Volcano plots of differentially expressed genes (left panels) and repetitive elements (right panels) in p150- (upper panels) and p60- (lower panels) depleted ES cells are shown. The data analysed derive from two independent biological replicates for all three conditions (within replicate correlation >= 0.97 for each replicate pair; Spearman’s rho). An MA representation of the same data is depicted in Figure 4a.
b. Venn diagram comparing up- and down-regulated genes in p150- or p60-depleted ES cells.
c. Venn diagram showing the comparison between differentially expressed genes in p150- or p60-depleted ES cells and those in 2-cell embryo. The differentially expressed genes in 2-cell stage embryo were obtained employing the same differential gene expression analysis as for the p150- and p60-depleted cells, comparing RNA-seq data from oocyte and 2-cell embryos from Macfarlan and colleagues (Macfarlan, A. et al., Nature. 487, 57-63, 2012).
d. Diagram showing the strategy for the distance analysis performed for Figure 4c. The genomic coordinates of a specific repeat type (Repeat X) were determined genome-wide, and windows of 500 bp upstream and downstream of each occurrence of the repeat X were used to record the genes located up to a +/- 200 kb distance from each repeat occurrence (different instances of Repeat X are depicted with subscripts). We then calculated the percentage of upregulated genes (green boxes) in p150- or p60-depleted cells among the total number of genes in a given interval.
e. Schematic representation for the distance analysis performed in Figure 4d. The genomic coordinates for the transcription start site of each set of upregulated genes were determined, and the distance to the closest end of either mt2_mm, mt2b1 or mt2c repeat was determined. Note that, in comparison to d, in which the analysis is ‘repeat centric’, the diagram depicted in e is ‘gene centric’.
Supplementary Figure 6 RNA-seq analyses on FACS-sorted cells.
a. MA plots displaying differentially expressed genes in untreated GFP-positive cells, GFP-positive cells after p150 KD and GFP-positive cells after p60 KD compared to untreated GFP-negative ES cells. Data is from 2 biological replicates (Spearman’s rho >= 0.95 for each pair of replicates; Supp. Tables 5 - 6).
b. Comparison of fold-change differential gene expression levels between endogenous 2C-like cells (untreated GFP-positive) and p150KD 2C-like cells (left) or p60KD 2C-like cells (right). To allow a direct comparison between the samples, all differential gene expression analyses in this figure are done against a common reference of FACS sorted untreated GFP-negative cells.
c. Venn diagram showing the comparison between differentially expressed genes in untreated GFP-positive cells, GFP-positive cells after p150 KD or GFP-positive cells after p60 KD and those in 2-cell embryo. Differentially expressed genes in 2-cell embryo obtained as in Supp. Fig.7c.
d. Distribution of distances (log2) between differentially expressed genes in untreated GFP-positive cells, GFP-positive cells after p150/p60 KD and/or genes upregulated in 2-cell embryos to 2-cell specific repeats. The absolute distance between the TSS of a gene and the end of the closest 2-cell specific MERVL family of repeat (either mt2_mm, mt2b1 or mt2c_mm) was measured for each gene within the dataset analysed. Box plots indicate the median log2 bp distance distribution and a confidence interval around it (notches). p-values calculated with Wilcoxon tests with Bonferroni correction for multiple testing.
e. Proportion of genes specific to each embryonic stage upregulated in endogenous 2-cell like, p60 KD induced 2-cell like and p150 KD induced 2-cell like cells. Genes specific to each stage were determined by comparison of datasets in Deng et al.,Science 343, 193-196 (2014). A gene was considered expressed when it showed an RPKM value higher than 10 in at least 20% of the cells analyzed for its respective stage (similar results were obtained for RKPM values of 1 and 5, and cell proportions between 10-50%). A gene was considered exclusive to a stage when it was found to be expressed in only a single stage out of: Zygote, mid-2-cell, 4-cell, 8-cell, 16-cell or early blastocyst. Sets of upregulated genes in endogenous 2-cell like, p60 KD induced 2-cell like and p150 KD induced 2-cell like cells were selected as in Supp. Fig.6c. Because the number of genes specific to each stage is different, we calculated the percentage of genes upregulated in each of the three 2C-like cells in relation to the number of gene subsets specific for each developmental stage. Statistical significance was assessed through Fisher’s exact tests with Bonferroni correction for multiple testing. p-values are as follows: in the comparison of endogenous 2C-like cells: with zygote p=0.724; with 2-cell p=2.901e-16; with 4-cell p=0.730; with 8-cell p=1; with 16-cell p=1; with early blastocyst p=0.373. In the comparison of p60KD-induced 2C–like cells: with zygote p=1; with 2-cell p=2.092e-13; with 4-cell p=0.051; with 8-cell p=1; with 16-cell p=1; with early blastocyst p=1. In the comparison of p150KD-induced 2C-like cells: with zygote p=1; with 2-cell p=4.448e-15; with 4-cell p=0.003; with 8-cell p=1; with 16-cell p=1; with early blastocyst p=1.
Supplementary Figure 7 Repeat analysis randomized, global chromatin accessibility in p60-induced 2C-like cells and SCNT raw data.
a. These data correspond to the same analysis strategy as in Figure 4d but with a random assignment of differentially expressed genes the same size as in the original groups; p150 KD (left), p60 KD (right). Coloured lines represent the percentage of randomly selected differentially expressed genes among all overlapping genes up to a distance of 200kb surrounding each repeat type, mt2_mm (blue), mt2b1 (green) and mt2c_mm (purple).
b. DNA fragment distribution after MNase digestion over increasing incubation timings in control, unsorted cells and EGFP-positive cells after p60 RNAi. After purification, the DNA was analyzed using a bioanalyzer. The purple and green lines indicate the position of the molecular weight marker in the chip. The DNA size corresponding to mono-, di- or tri-nucleosome is indicated on the right of each panel.
c. Raw data from nuclear transfer experiments with untreated GFP-negative (GFP-), untreated GFP-positive (GFP+) and GFP-positive cells after p60 KD (sip60 GFP+) are summarized in the table.
d. Comparison of SCNT efficiency using unsorted ES cells (no FACS) and GFP- negative ES cells. The results of nuclear transfer experiment with FACS-sorted GFP-negative cells (GFP-) and unsorted cells (No FACS) are summarized in the table. Given that the population of GFP-negative cells is ~99% in ES cells, the difference between these two groups is due to the effect of FACS procedure itself. For c and d: PN, number of NT-embryos with pronuclear formation; 1&ab, number of NT-embryos arrested at 1-cell or showing abnormal morphology; 2C, number of NT-embryos developed to 2-cell stage; 4/8C, number of NT-embryos developed to 4- or 8-cell stage; M/B, number of NT-embryos developed to morula or blastocyst. The percent of the development to 2-cell (2cell%), 4- or 8-cell (4/8C%), and morula or blastocyst (M/B%) was calculated using the number of NT-embryos that formed visible pronuclei.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–7 and Supplementary Note (PDF 21288 kb)
Supplementary Data Set 1
Uncropped images for the blots shown in Figure 1, Figure 3, Supplementary Figure 2 and Supplementary Figure 4 (PDF 173 kb)
Supplementary Table 1
List of differentially expressed genes in ES cells after p150 RNAi (XLS 4804 kb)
Supplementary Table 2
List of differentially expressed genes in ES cells after p60 RNAi (XLS 4679 kb)
Supplementary Table 3
List of differentially expressed repeats in ES cells after p150 RNAi (XLS 226 kb)
Supplementary Table 4
Lists of differentially expressed repeats in ES cells after p60 RNAi (XLS 226 kb)
Supplementary Table 5
List of differentially expressed genes in sorted GFP-, GFP+, sicontol-GFP+ and GFP(+) after p150/p60 RNAi (XLSX 12326 kb)
Supplementary Table 6
List of differentially expressed repeats in sorted GFP-, GFP+, sicontol-GFP+ and GFP(+) after p150/p60 RNAi (XLSX 562 kb)
Supplementary Table 7
List of common genes upregulated in p150 RNAi and expressed in 2-cell-stage embryos (XLS 56 kb)
Supplementary Table 8
List of common genes upregulated in p60 RNAi and expressed in 2-cell-stage embryos (XLS 46 kb)
Supplementary Table 9
Total number of reads included in the different analyses (XLSX 51 kb)
Rights and permissions
About this article
Cite this article
Ishiuchi, T., Enriquez-Gasca, R., Mizutani, E. et al. Early embryonic-like cells are induced by downregulating replication-dependent chromatin assembly. Nat Struct Mol Biol 22, 662–671 (2015). https://doi.org/10.1038/nsmb.3066
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nsmb.3066
This article is cited by
-
Quiescence enables unrestricted cell fate in naive embryonic stem cells
Nature Communications (2024)
-
The homeobox transcription factor DUXBL controls exit from totipotency
Nature Genetics (2024)
-
Symmetric inheritance of parental histones governs epigenome maintenance and embryonic stem cell identity
Nature Genetics (2023)
-
Nucleolus assembly impairment leads to two-cell transcriptional repression via NPM1-mediated PRC2 recruitment
Nature Structural & Molecular Biology (2023)
-
Histone chaperone HIRA complex regulates retrotransposons in embryonic stem cells
Stem Cell Research & Therapy (2022)