Abstract
Retrotransposons are a class of mobile genetic elements that replicate by converting their single-stranded RNA intermediate to double-stranded DNA through the combined DNA polymerase and ribonuclease H (RNase H) activities of the element-encoded reverse transcriptase (RT). Although a wealth of structural information is available for lentiviral and gammaretroviral RTs, equivalent studies on counterpart enzymes of long terminal repeat (LTR)–containing retrotransposons, from which they are evolutionarily derived, is lacking. In this study, we report the first crystal structure of a complex of RT from the Saccharomyces cerevisiae LTR retrotransposon Ty3 in the presence of its polypurine tract–containing RNA-DNA hybrid. In contrast to its retroviral counterparts, Ty3 RT adopts an asymmetric homodimeric architecture whose assembly is substrate dependent. Moreover, our structure and biochemical data suggest that the RNase H and DNA polymerase activities are contributed by individual subunits of the homodimer.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Cordaux, R. & Batzer, M.A. The impact of retrotransposons on human genome evolution. Nat. Rev. Genet. 10, 691–703 (2009).
Lander, E.S. et al. Initial sequencing and analysis of the human genome. Nature 409, 860–921 (2001).
SanMiguel, P., Gaut, B.S., Tikhonov, A., Nakajima, Y. & Bennetzen, J.L. The paleontology of intergene retrotransposons of maize. Nat. Genet. 20, 43–45 (1998).
Malik, H.S., Henikoff, S. & Eickbush, T.H. Poised for contagion: evolutionary origins of the infectious abilities of invertebrate retroviruses. Genome Res. 10, 1307–1318 (2000).
Llorens, C. et al. The Gypsy Database (GyDB) of mobile genetic elements: release 2.0. Nucleic Acids Res. 39, D70–D74 (2011).
Sandmeyer, S.B., Aye, M. & Menees, T.M. Ty3: a position-specific, gypsylike element in Saccharomyces cerevisiae. in Mobile DNA II (eds. Craig, N.L., Craigie, R., Gellert, M. & Lambowitz, A.M.) 663–682 (ASM Press, Washington, DC, 2002).
Bibillo, A., Lener, D., Klarmann, G.J. & Le Grice, S.F. Functional roles of carboxylate residues comprising the DNA polymerase active site triad of Ty3 reverse transcriptase. Nucleic Acids Res. 33, 171–181 (2005).
Bibillo, A., Lener, D., Tewari, A. & Le Grice, S.F. Interaction of the Ty3 reverse transcriptase thumb subdomain with template-primer. J. Biol. Chem. 280, 30282–30290 (2005).
Lener, D., Budihas, S.R. & Le Grice, S.F. Mutating conserved residues in the ribonuclease H domain of Ty3 reverse transcriptase affects specialized cleavage events. J. Biol. Chem. 277, 26486–26495 (2002).
Brinson, R.G. et al. Probing anomalous structural features in polypurine tract-containing RNA-DNA hybrids with neomycin B. Biochemistry 48, 6988–6997 (2009).
Dash, C., Marino, J.P. & Le Grice, S.F. Examining Ty3 polypurine tract structure and function by nucleoside analog interference. J. Biol. Chem. 281, 2773–2783 (2006).
Rausch, J.W. et al. Interaction of p55 reverse transcriptase from the Saccharomyces cerevisiae retrotransposon Ty3 with conformationally distinct nucleic acid duplexes. J. Biol. Chem. 275, 13879–13887 (2000).
Turner, K.B. et al. SHAMS: combining chemical modification of RNA with mass spectrometry to examine polypurine tract-containing RNA/DNA hybrids. RNA 15, 1605–1613 (2009).
Yi-Brunozzi, H.Y., Brabazon, D.M., Lener, D., Le Grice, S.F. & Marino, J.P. A ribose sugar conformational switch in the LTR-retrotransposon Ty3 polypurine tract-containing RNA/DNA hybrid. J. Am. Chem. Soc. 127, 16344–16345 (2005).
Yi-Brunozzi, H.Y. et al. High-resolution NMR analysis of the conformations of native and base analog substituted retroviral and LTR-retrotransposon PPT primers. Chem. Biol. 15, 254–262 (2008).
Huang, H., Chopra, R., Verdine, G.L. & Harrison, S.C. Structure of a covalently trapped catalytic complex of HIV-1 reverse transcriptase: implications for drug resistance. Science 282, 1669–1675 (1998).
Lapkouski, M., Tian, L., Miller, J.T., Le Grice, S.F. & Yang, W. Complexes of HIV-1 RT, NNRTI and RNA/DNA hybrid reveal a structure compatible with RNA degradation. Nat. Struct. Mol. Biol. 20, 230–236 (2013).
Malik, H.S. & Eickbush, T.H. Phylogenetic analysis of ribonuclease H domains suggests a late, chimeric origin of LTR retrotransposable elements and retroviruses. Genome Res. 11, 1187–1197 (2001).
Kirchner, J. & Sandmeyer, S.B. Ty3 integrase mutants defective in reverse transcription or 3′-end processing of extrachromosomal Ty3 DNA. J. Virol. 70, 4737–4747 (1996).
Wilhelm, M., Heyman, T., Friant, S. & Wilhelm, F.X. Heterogeneous terminal structure of Ty1 and Ty3 reverse transcripts. Nucleic Acids Res. 25, 2161–2166 (1997).
Wilhelm, M., Uzun, O., Mules, E.H., Gabriel, A. & Wilhelm, F.X. Polypurine tract formation by Ty1 RNase H. J. Biol. Chem. 276, 47695–47701 (2001).
Nymark-McMahon, M.H., Beliakova-Bethell, N.S., Darlix, J.L., Le Grice, S.F. & Sandmeyer, S.B. Ty3 integrase is required for initiation of reverse transcription. J. Virol. 76, 2804–2816 (2002).
Nowak, E. et al. Structural analysis of monomeric retroviral reverse transcriptase in complex with an RNA/DNA hybrid. Nucleic Acids Res. 41, 3874–3887 (2013).
Kohlstaedt, L.A., Wang, J., Friedman, J.M., Rice, P.A. & Steitz, T.A. Crystal structure at 3.5 A resolution of HIV-1 reverse transcriptase complexed with an inhibitor. Science 256, 1783–1790 (1992).
Mous, J., Heimer, E.P. & Le Grice, S.F. Processing protease and reverse transcriptase from human immunodeficiency virus type I polyprotein in Escherichia coli. J. Virol. 62, 1433–1436 (1988).
Nowotny, M. Retroviral integrase superfamily: the structural perspective. EMBO Rep. 10, 144–151 (2009).
Nowotny, M., Gaidamakov, S.A., Crouch, R.J. & Yang, W. Crystal structures of RNase H bound to an RNA/DNA hybrid: substrate specificity and metal-dependent catalysis. Cell 121, 1005–1016 (2005).
Nowotny, M. et al. Structure of human RNase H1 complexed with an RNA/DNA hybrid: insight into HIV reverse transcription. Mol. Cell 28, 264–276 (2007).
Najmudin, S. et al. Crystal structures of an N-terminal fragment from Moloney murine leukemia virus reverse transcriptase complexed with nucleic acid: functional implications for template-primer binding to the fingers domain. J. Mol. Biol. 296, 613–632 (2000).
Boyer, P.L., Ferris, A.L. & Hughes, S.H. Cassette mutagenesis of the reverse transcriptase of human immunodeficiency virus type 1. J. Virol. 66, 1031–1039 (1992).
Kim, B. et al. New human immunodeficiency virus, type 1 reverse transcriptase (HIV-1 RT) mutants with increased fidelity of DNA synthesis. Accuracy, template binding, and processivity. J. Biol. Chem. 274, 27666–27673 (1999).
Sarafianos, S.G. et al. Crystal structure of HIV-1 reverse transcriptase in complex with a polypurine tract RNA:DNA. EMBO J. 20, 1449–1461 (2001).
Beard, W.A. et al. Structure/function studies of human immunodeficiency virus type 1 reverse transcriptase. Alanine scanning mutagenesis of an alpha-helix in the thumb subdomain. J. Biol. Chem. 269, 28091–28097 (1994).
Bebenek, K. et al. Reduced frameshift fidelity and processivity of HIV-1 reverse transcriptase mutants containing alanine substitutions in helix H of the thumb subdomain. J. Biol. Chem. 270, 19516–19523 (1995).
Bebenek, K. et al. A minor groove binding track in reverse transcriptase. Nat. Struct. Biol. 4, 194–197 (1997).
Powell, M.D. et al. Residues in the αH and αI helices of the HIV-1 reverse transcriptase thumb subdomain required for the specificity of RNase H-catalyzed removal of the polypurine tract primer. J. Biol. Chem. 274, 19885–19893 (1999).
North, T.W., Cronn, R.C., Remington, K.M., Tandberg, R.T. & Judd, R.C. Characterization of reverse transcriptase from feline immunodeficiency virus. J. Biol. Chem. 265, 5121–5128 (1990).
Thomas, D.A. & Furman, P.A. Purification and kinetic characterization of equine infectious anemia virus reverse transcriptase. Biochem. Biophys. Res. Commun. 180, 1365–1371 (1991).
Rausch, J.W. & Le Grice, S.F. 'Binding, bending and bonding': polypurine tract-primed initiation of plus-strand DNA synthesis in human immunodeficiency virus. Int. J. Biochem. Cell Biol. 36, 1752–1766 (2004).
Swapna, L.S., Srikeerthana, K. & Srinivasan, N. Extent of structural asymmetry in homodimeric proteins: prevalence and relevance. PLoS ONE 7, e36688 (2012).
Karplus, P.A. & Diederichs, K. Linking crystallographic model and data quality. Science 336, 1030–1033 (2012).
Kabsch, W. Xds. Acta Crystallogr. D Biol. Crystallogr. 66, 125–132 (2010).
Afonine, P.V., Grosse-Kunstleve, R.W. & Adams, P.D. The Phenix refinement framework. CCP4 Newsl. 42, contribution 8 (2005).
Emsley, P. & Cowtan, K. Coot: model-building tools for molecular graphics. Acta Crystallogr. D Biol. Crystallogr. 60, 2126–2132 (2004).
Schuck, P. Size-distribution analysis of macromolecules by sedimentation velocity ultracentrifugation and lamm equation modeling. Biophys. J. 78, 1606–1619 (2000).
Acknowledgements
We thank W. Yang for critical reading of the manuscript, I. Ptasiewicz for excellent technical assistance, the staff of beamline 14-1 at Berliner Elektronenspeicherring-Gesellschaft für Synchrotronstrahlung for assistance with data collection, and J.M. Bujnicki for help in the preparation of the sequence alignments. This work was supported by grants from the Polish National Science Center (contract no. N N301 439738 to M.N.) and the FP7 HEALTHPROT project (contract no. 229676 to M.N.). S.F.J.L.G. is supported by the Intramural Research Program of the National Cancer Institute, US National Institutes of Health and by federal funds from National Institutes of Health contract no. HHSN261200800001E (M.K.B.). The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products or organizations imply endorsement by the US Government. M.N. is a recipient of the Foundation for Polish Science 'Ideas for Poland' award. The research of M.N. was supported in part by an International Early Career Scientist grant from the Howard Hughes Medical Institute. The research was performed using Centre for Preclinical Research and Technology infrastructure (European Union project POIG.02.02.00-14-024/08-00).
Author information
Authors and Affiliations
Contributions
E.N. obtained crystals of Ty3 RT–substrate complex; E.N. and M.N. solved and analyzed the structure; J.T.M., M.K.B. and J.S. performed biochemical experiments; E.N. and R.H.S. performed biophysical protein characterization; J.J. prepared the expression construct and conducted initial crystallization experiments; S.F.J.L.G. and M.N. designed the research and wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Integrated supplementary information
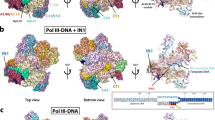
Supplementary Figure 1 Experimental electron density maps and the content of the asymmetric unit.
(a) and (b) Samples of experimental electron density maps after selenium single wavelength anomalous diffraction (SAD) phasing (stereoviews). (a) The region around the DNA polymerase active site. (b) The central β-sheet of RNase H domain from subunit B. Maps are contoured at 1.5 and 1.2 σ. (c) Composition of the asymmetric unit. The two complexes present in the asymmetric unit are shown, colored as in Figure 1.
Supplementary Figure 2 Multiple sequence alignment of retrolement and retrovirus RT sequences.
Ty3/Gypsy retroelements: Boudicca (S. mansoni), Gypsy (D. melanogaster), Maggy (M. grisea), Ulysses (D. virilis), Real (A. alternata), TF2 (S. pombe), Ty3 and retroviruses: human immunodeficiency virus 1 (HIV-1), xenotropic murine leukemia-related virus (XMRV), human T-cell leukemia virus-1 (HTLV-I), prototype foamy virus (PFV), Rous sarcoma virus (RSV), mouse mammary tumor virus (MMTV). Retroelements were selected to represent the most diverse sequences based on phylogenetic analysis of the RT sequence. For all sequences the active site residues are highlighted in yellow. For Ty3 RT sequence residues involved in RNA stabilization are highlighted in red, and DNA interaction in blue. Dimer interface residues are highlighted in gray. Numbers on the top of the alignment correspond to Ty3 RT residues.
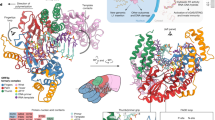
Supplementary Figure 3 Structure-based alignment of RTs from Ty3 and HIV-1 (PDB ID: 1RTD16).
Identical residues are marked with '*' and similar ones with ':'. Secondary structure elements are shown as tubes (helices) and arrows (strands) and labeled and colored as in Figure 1. Part of the C-terminal region of Ty3 RNase H domain adopts different conformation in subunits A and B and therefore, two secondary structure assignments are given and fragments not observed in the structure are marked with '~'. Active site residues of the DNA polymerase and RNase H (for Ty3) domains are highlighted in yellow. Note the similarity in structural organization between the HIV-1 RT p66 connection subdomain and the Ty3 RNase H domain.
Supplementary Figure 4 Structure-based alignment of RNase H domains from HIV-1 and Ty3 RTs.
The alignment is marked as in Supplementary Figure 3 and secondary structure elements labeled as in Fig 1 and 2.
Supplementary Figure 5 RNase H active sites and model of the interaction with the substrate.
(a) Comparison of RNase H active sites (stereoview) shown in yellow (Ty3 RT), blue (B. halodurans RNase H1, PDB ID: 1ZBI27), and salmon (human RNase H1 PDB ID: 2QK9 (ref 28)). Two Mg2+ ions in the Bh-RNase H1 structure are shown as green spheres and the RNA strands from human and bacterial RNases H1 in salmon and red, respectively. The attacking nucleophilic water is shown as a red sphere. (b) Model of the interaction of the Ty3 subunit B RNase H domain with the scissile phosphate. RNase H domains and the nucleic acid are in cartoon representation and the rest of the structure in wire representation. Subunit A is shown in dark gray and yellow for RNase H and subunit B in light gray and light yellow for RNase H. Brown cartoon represents the RNase H domain modeled to interact with the scissile phosphate in position –13. Model with 12 nt distance from the 3′ end of the DNA strand was equally plausible and did not to include any steric clashes. RNA is in magenta and DNA in blue.
Supplementary Figure 6 Comparison of the DNA polymerase active sites of Ty3 and HIV-1 RT (PDB ID: 1RTD16) (stereoview).
Ty3 RT residues are shown in red (palm) and blue (fingers) and HIV-1 in grey. Metal ions and the incoming dNTP from HIV-1 structure are shown in gray. RNA-DNA from Ty3 structure is shown in magenta (RNA) and marine (DNA) ladder. dsDNA from the HIV-1 structure is shown as light blue ladder. The last nucleotide of the DNA located at the active site is shown with sticks.
Supplementary Figure 7 Substrate binding an oligomerization of Ty3 RT variants – analytical ultracentrifugation, sedimentation velocity analysis.
(a) Black trace - RNA-DNA hybrid alone, calculated MW of 18.8 kDa (assuming v-bar =0.54 ml g-1, value the most commonly used for nucleic acids), s=1.88 S, value normalized for 20 °C and water s20,w =1.74 S. Red - R140A R203A variant alone, MW of 49.1 (v-bar 0.7352), s =2.2 S and s20,w = 3.5 S. (b) Magenta - RNA-DNA with R140A R203A, estimated MW of 86 kDa, s= 3.5 S and s20,w =5.6 S. Green - DNA-RNA with R441A R442A, MW of 127 kDa, s= 4.2 S and s20,w =6.6 S. Blue – DNA-RNA with WT, estimated MW of 121 kDa, s =4.3 S and normalized s20,w =6.9 S. Excess of unbound proteins, WT and R441A R442A variant, with the MW of 56 kDa and 57 kDa, respectively, are marked with corresponding colors. The shift in the position of the peak for R441A R442A may be a result of a conformational change relative to the wild type protein.
Supplementary Figure 8 Uncropped images used in Fig. 4c and d.
(a) Ty3 RT DNA polymerase assay on HIV-1 RNA genome. The lanes used in Fig 4c are indicated with a blue line in the bottom of the gel. P-fluorescently labeled primer. (b) A sequencing reference gel with the reaction for HIV-1 RT used to identify the polymerase reaction products. dA – sequencing ladder, Mut – RNase H-deficient HIV-1 RT, WT – wild type HIV-1 RT. The position of the TAR hairpin (TAR), which causes stalling of the Ty3 RT is indicated on the left of the gel. (c) Uncropped gel presented in Fig 4d. (d) A reference Ty3 RT RNase H assay. Wild type protein was incubated with a fluorescently labeled hybrid and the reaction was stopped after 0.5, 10 and 20 minutes (indicated on top of the gel). Three different amounts of RNA size markers with the same fluorescent label were applied to the lanes 'markers'. Their sizes are indicated and the corresponding cut positions from the 3′-end of the DNA are given in parentheses.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–8 and Supplementary Note (PDF 2001 kb)
Rights and permissions
About this article
Cite this article
Nowak, E., Miller, J., Bona, M. et al. Ty3 reverse transcriptase complexed with an RNA-DNA hybrid shows structural and functional asymmetry. Nat Struct Mol Biol 21, 389–396 (2014). https://doi.org/10.1038/nsmb.2785
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nsmb.2785
This article is cited by
-
Orchestrating nucleic acid–protein interactions at chromosome ends: telomerase mechanisms come into focus
Nature Structural & Molecular Biology (2023)
-
Convergence of retrotransposons in oomycetes and plants
Mobile DNA (2017)
-
Transcriptionally active LTR retrotransposons in Eucalyptus genus are differentially expressed and insertionally polymorphic
BMC Plant Biology (2015)