Abstract
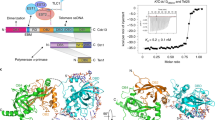
The Ever shorter telomeres 3 (Est3) protein is a small regulatory subunit of yeast telomerase which is dispensable for enzyme catalysis but essential for telomere replication in vivo. Using structure prediction combined with in vivo characterization, we show here that Est3 consists of a predicted OB (oligosaccharide/oligonucleotide binding)-fold. We used mutagenesis of predicted surface residues to generate a functional map of one surface of Est3, identifying a site that mediates association with the telomerase complex. Unexpectedly, the predicted OB-fold of Est3 is structurally similar to the OB-fold of the human TPP1 protein, despite the fact that Est3 and TPP1, as components of telomerase and a telomere-capping complex, respectively, perform functionally distinct tasks at chromosome ends. Our analysis of Est3 may be instructive in generating comparable missense mutations on the surface of the OB-fold domain of TPP1.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Garcia, C.K., Wright, W.E. & Shay, J.W. Human diseases of telomerase dysfunction: insights into tissue aging. Nucleic Acids Res. 35, 7406–7416 (2007).
Singer, M.S. & Gottschling, D.E. TLC1: template RNA component of Saccharomyces cerevisiae telomerase. Science 266, 404–409 (1994).
Lingner, J. et al. Reverse transcriptase motifs in the catalytic subunit of telomerase. Science 276, 561–567 (1997).
Hughes, T.R., Evans, S.K., Weilbaecher, R.G. & Lundblad, V. The Est3 protein is a subunit of yeast telomerase. Curr. Biol. 10, 809–812 (2000).
Zappulla, D.C. & Cech, T.R. Yeast telomerase RNA: a flexible scaffold for protein subunits. Proc. Natl. Acad. Sci. USA 101, 10024–10029 (2004).
Lendvay, T.S., Morris, D.K., Sah, J., Balasubramanian, B. & Lundblad, V. Senescence mutants of Saccharomyces cerevisiae with a defect in telomere replication identify three additional EST genes. Genetics 144, 1399–1412 (1996).
Cohn, M. & Blackburn, E.H. Telomerase in yeast. Science 269, 396–400 (1995).
Lingner, J., Cech, T.R., Hughes, T.R. & Lundblad, V. Three Ever Shorter Telomere (EST) genes are dispensable for in vitro yeast telomerase activity. Proc. Natl. Acad. Sci. USA 94, 11190–11195 (1997).
Beernink, H.T., Miller, K., Deshpande, A., Bucher, P. & Cooper, J.P. Telomere maintenance in fission yeast requires an Est1 ortholog. Curr. Biol. 13, 575–580 (2003).
Leonardi, J., Box, J.A., Bunch, J.T. & Baumann, P. TER1, the RNA subunit of fission yeast telomerase. Nat. Struct. Mol. Biol. 15, 26–33 (2008).
Livengood, A.J., Zaug, A.J. & Cech, T.R. Essential regions of Saccharomyces cerevisiae telomerase RNA: separate elements for Est1p and Est2p interaction. Mol. Cell. Biol. 22, 2366–2374 (2002).
Seto, A.G., Livengood, A.J., Tzfati, Y., Blackburn, E.H. & Cech, T.R. A bulged stem tethers Est1p to telomerase RNA in budding yeast. Genes Dev. 16, 2800–2812 (2002).
Webb, C.J. & Zakian, V.A. Identification and characterization of the Schizosaccharomyces pombe TER1 telomerase RNA. Nat. Struct. Mol. Biol. 15, 34–42 (2008).
Nugent, C.I., Hughes, T.R., Lue, N.F. & Lundblad, V. Cdc13p: a single-strand telomeric DNA-binding protein with a dual role in yeast telomere maintenance. Science 274, 249–252 (1996).
Pennock, E., Buckley, K. & Lundblad, V. Cdc13 delivers separate complexes to the telomere for end protection and replication. Cell 104, 387–396 (2001).
Bianchi, A., Negrini, S. & Shore, D. Delivery of yeast telomerase to a DNA break depends on the recruitment functions of Cdc13 and Est1. Mol. Cell 16, 139–146 (2004).
Bianchi, A. & Shore, D. Increased association of telomerase with short telomeres in yeast. Genes Dev. 21, 1726–1730 (2007).
Teixeira, M.T., Arneric, M., Sperisen, P. & Lingner, J. Telomere length homeostasis is achieved via a switch between telomerase-extendible and -nonextendible states. Cell 117, 323–335 (2004).
Taggart, A.K., Teng, S.C. & Zakian, V.A. Est1p as a cell cycle-regulated activator of telomere-bound telomerase. Science 297, 1023–1026 (2002).
Friedman, K.L., Heit, J.J., Long, D.M. & Cech, T.R. N-terminal domain of yeast telomerase reverse transcriptase: recruitment of Est3p to the telomerase complex. Mol. Biol. Cell 14, 1–13 (2003).
Hsu, M., Yu, E.Y., Singh, S.M. & Lue, N.F. Mutual dependence of Candida albicans Est1p and Est3p in telomerase assembly and activation. Eukaryot. Cell 6, 1330–1338 (2007).
Osterhage, J.L., Talley, J.M. & Friedman, K.L. Proteasome-dependent degradation of Est1p regulates the cell cycle-restricted assembly of telomerase in Saccharomyces cerevisiae. Nat. Struct. Mol. Biol. 13, 720–728 (2006).
Fang, G.W. & Cech, T.R. Molecular cloning of telomere-binding protein genes from Stylonychia mytilis. Nucleic Acids Res. 19, 5515–5518 (1991).
Gray, J.T., Celander, D.W., Price, C.M. & Cech, T.R. Cloning and expression of genes for the Oxytricha telomere-binding protein: specific subunit interactions in the telomeric complex. Cell 67, 807–814 (1991).
Baumann, P. & Cech, T.R. Pot1, the putative telomere end-binding protein in fission yeast and humans. Science 292, 1171–1175 (2001).
Ye, J.Z. et al. POT1-interacting protein PIP1: a telomere length regulator that recruits POT1 to the TIN2/TRF1 complex. Genes Dev. 18, 1649–1654 (2004).
Liu, D. et al. PTOP interacts with POT1 and regulates its localization to telomeres. Nat. Cell Biol. 6, 673–680 (2004).
Houghtaling, B.R., Cuttonaro, L., Chang, W. & Smith, S. A dynamic molecular link between the telomere length regulator TRF1 and the chromosome end protector TRF2. Curr. Biol. 14, 1621–1631 (2004).
Churikov, D., Wei, C. & Price, C.M. Vertebrate POT1 restricts G-overhang length and prevents activation of a telomeric DNA damage checkpoint but is dispensable for overhang protection. Mol. Cell. Biol. 26, 6971–6982 (2006).
Jacob, N.K., Lescasse, R., Linger, B.R. & Price, C.M. Tetrahymena POT1a regulates telomere length and prevents activation of a cell cycle checkpoint. Mol. Cell. Biol. 27, 1592–1601 (2007).
Hockemeyer, D. et al. Telomere protection by mammalian Pot1 requires interaction with Tpp1. Nat. Struct. Mol. Biol. 14, 754–761 (2007).
Guo, X. et al. Dysfunctional telomeres activate an ATM-ATR-dependent DNA damage response to suppress tumorigenesis. EMBO J. 26, 4709–4719 (2007).
Else, T. et al. Tpp1/Acd maintains genomic stability through a complex role in telomere protection. Chromosome Res. 15, 1001–1013 (2007).
Miyoshi, T., Kanoh, J., Saito, M. & Ishikawa, F. Fission yeast Pot1-Tpp1 protects telomeres and regulates telomere length. Science 320, 1341–1344 (2008).
Morris, D.K. & Lundblad, V. Programmed translational frameshifting in a gene required for yeast telomere replication. Curr. Biol. 7, 969–976 (1997).
Singh, S.M., Steinberg-Neifach, O., Mian, I.S. & Lue, N.F. Analysis of telomerase in Candida albicans: potential role in telomere end protection. Eukaryot. Cell 1, 967–977 (2002).
Soding, J., Biegert, A. & Lupas, A.N. The HHpred interactive server for protein homology detection and structure prediction. Nucleic Acids Res. 33, W244–W248 (2005).
Wang, F. et al. The POT1–TPP1 telomere complex is a telomerase processivity factor. Nature 445, 506–510 (2007).
Karplus, K. et al. SAM-T04: what is new in protein-structure prediction for CASP6. Proteins 61, S135–S142 (2005).
Shi, J., Blundell, T.L. & Mizuguchi, K. FUGUE: sequence-structure homology recognition using environment-specific substitution tables and structure-dependent gap penalties. J. Mol. Biol. 310, 243–257 (2001).
Horvath, M.P., Schweiker, V.L., Bevilacqua, J.M., Ruggles, J.A. & Schultz, S.C. Crystal structure of the Oxytricha nova telomere end binding protein complexed with single strand DNA. Cell 95, 963–974 (1998).
Zhang, Y. I-TASSER server for protein 3D structure prediction. BMC Bioinformatics 9, 40 (2008).
Evans, S.K. & Lundblad, V. The Est1 subunit of Saccharomyces cerevisiae telomerase makes multiple contributions to telomere length maintenance. Genetics 162, 1101–1115 (2002).
Xin, H. et al. TPP1 is a homologue of ciliate TEBP-β and interacts with POT1 to recruit telomerase. Nature 445, 559–562 (2007).
Singh, S.M. & Lue, N.F. Ever shorter telomere 1 (EST1)-dependent reverse transcription by Candida telomerase in vitro: evidence in support of an activating function. Proc. Natl. Acad. Sci. USA 100, 5718–5723 (2003).
Armougom, F. et al. Expresso: automatic incorporation of structural information in multiple sequence alignments using 3D-Coffee. Nucleic Acids Res. 34, W604–W608 (2006).
Pei, J., Kim, B.H., Tang, M. & Grishin, N.V. PROMALS web server for accurate multiple protein sequence alignments. Nucleic Acids Res. 35, W649–W652 (2007).
Moretti, S. et al. The M-Coffee web server: a meta-method for computing multiple sequence alignments by combining alternative alignment methods. Nucleic Acids Res. 35, W645–W648 (2007).
Sali, A. & Blundell, T.L. Comparative protein modelling by satisfaction of spatial restraints. J. Mol. Biol. 234, 779–815 (1993).
Holm, L. & Park, J. DaliLite workbench for protein structure comparison. Bioinformatics 16, 566–567 (2000).
Acknowledgements
We thank L. Ricks and E. Ford for outstanding technical assistance. This research was supported by grant AG11728 from the US National Institutes of Health.
Author information
Authors and Affiliations
Corresponding author
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–4 and Supplmentary Table 1 (PDF 5350 kb)
Rights and permissions
About this article
Cite this article
Lee, J., Mandell, E., Tucey, T. et al. The Est3 protein associates with yeast telomerase through an OB-fold domain. Nat Struct Mol Biol 15, 990–997 (2008). https://doi.org/10.1038/nsmb.1472
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nsmb.1472
This article is cited by
-
Orchestrating nucleic acid–protein interactions at chromosome ends: telomerase mechanisms come into focus
Nature Structural & Molecular Biology (2023)
-
Insights into the structure and function of Est3 from the Hansenula polymorpha telomerase
Scientific Reports (2020)
-
Fine tuning the level of the Cdc13 telomere-capping protein for maximal chromosome stability performance
Current Genetics (2019)
-
Chemical shift assignments and the secondary structure of the Est3 telomerase subunit in the yeast Hansenula polymorpha
Biomolecular NMR Assignments (2018)
-
Proteomics of yeast telomerase identified Cdc48-Npl4-Ufd1 and Ufd4 as regulators of Est1 and telomere length
Nature Communications (2015)