Abstract
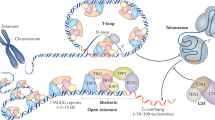
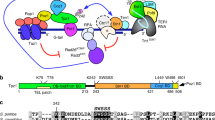
Budding yeast Cdc13–Stn1–Ten1 (CST) complex plays an essential role in telomere protection and maintenance. Despite extensive studies, only structural information of individual domains of CST is available; the architecture of CST still remains unclear. Here, we report crystal structures of Kluyveromyces lactis Cdc13–telomeric-DNA, Cdc13–Stn1 and Stn1–Ten1 complexes and propose an integrated model depicting how CST assembles and plays its roles at telomeres. Surprisingly, two oligonucleotide/oligosaccharide-binding (OB) folds of Cdc13 (OB2 and OB4), previously believed to mediate Cdc13 homodimerization, actually form a stable intramolecular interaction. This OB2–OB4 module of Cdc13 is required for the Cdc13–Stn1 interaction that assembles CST into an architecture with a central ring-like core and multiple peripheral modules in a 2:2:2 stoichiometry. Functional analyses indicate that this unique CST architecture is essential for both telomere capping and homeostasis regulation. Overall, our results provide fundamentally valuable structural information regarding the CST complex and its roles in telomere biology.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
McEachern, M. J., Krauskopf, A. & Blackburn, E. H. Telomeres and their control. Annu. Rev. Genet. 34, 331–358 (2000).
Stewart, J. A., Chaiken, M. F., Wang, F. & Price, C. M. Maintaining the end: roles of telomere proteins in end-protection, telomere replication and length regulation. Mutat. Res. 730, 12–19 (2012).
Pfeiffer, V. & Lingner, J. Replication of telomeres and the regulation of telomerase. Cold Spring Harb. Perspect. Biol. 5, a010405 (2013).
Soudet, J., Jolivet, P. & Teixeira, M. T. Elucidation of the DNA end-replication problem in Saccharomyces cerevisiae. Mol. Cell 53, 954–964 (2014).
Palm, W. & de Lange, T. How shelterin protects mammalian telomeres. Annu. Rev. Genet. 42, 301–334 (2008).
Cervantes, R. B. & Lundblad, V. Mechanisms of chromosome-end protection. Curr. Opin. Cell Biol. 14, 351–356 (2002).
Zakian, V. A. Telomeres: beginning to understand the end. Science 270, 1601–1607 (1995).
McEachern, M. J. & Blackburn, E. H. A conserved sequence motif within the exceptionally diverse telomeric sequences of budding yeasts. Proc. Natl Acad. Sci. USA 91, 3453–3457 (1994).
Greider, C. W. & Blackburn, E. H. Identification of a specific telomere terminal transferase activity in Tetrahymena extracts. Cell 43, 405–413 (1985).
Jiang, J. et al. Structure of Tetrahymena telomerase reveals previously unknown subunits, functions, and interactions. Science 350, aab4070 (2015).
Zappulla, D. C. & Cech, T. R. Yeast telomerase RNA: a flexible scaffold for protein subunits. Proc. Natl Acad. Sci. USA 101, 10024–10029 (2004).
Zappulla, D. C. et al. Ku can contribute to telomere lengthening in yeast at multiple positions in the telomerase RNP. RNA 17, 298–311 (2011).
Mefford, M. A., Rafiq, Q. & Zappulla, D. C. RNA connectivity requirements between conserved elements in the core of the yeast telomerase RNP. EMBO J. 32, 2980–2993 (2013).
Lemieux, B. et al. Active yeast telomerase shares subunits with ribonucleoproteins RNase P and RNase MRP. Cell 165, 1171–1181 (2016).
Wellinger, R. J. & Zakian, V. A. Everything you ever wanted to know about Saccharomyces cerevisiae telomeres: beginning to end. Genetics 191, 1073–1105 (2012).
Mitton-Fry, R. M., Anderson, E. M., Hughes, T. R., Lundblad, V. & Wuttke, D. S. Conserved structure for single-stranded telomeric DNA recognition. Science 296, 145–147 (2002).
Pennock, E., Buckley, K. & Lundblad, V. Cdc13 delivers separate complexes to the telomere for end protection and replication. Cell 104, 387–396 (2001).
Mersaoui, S. Y. & Wellinger, R. J. Fine tuning the level of the Cdc13 telomere-capping protein for maximal chromosome stability performance. Curr. Genet. 65, 109–118 (2019).
Mitchell, M. T. et al. Cdc13 N-terminal dimerization, DNA binding, and telomere length regulation. Mol. Cell. Biol. 30, 5325–5334 (2010).
Sun, J. et al. Structural bases of dimerization of yeast telomere protein Cdc13 and its interaction with the catalytic subunit of DNA polymerase ɑ. Cell Res. 21, 258–274 (2011).
Chen, H. et al. Structural insights into yeast telomerase recruitment to telomeres. Cell 172, 331–343.e13 (2018).
Mitton-Fry, R. M., Anderson, E. M., Theobald, D. L., Glustrom, L. W. & Wuttke, D. S. Structural basis for telomeric single-stranded DNA recognition by yeast Cdc13. J. Mol. Biol. 338, 241–255 (2004).
Mason, M. et al. Cdc13 OB2 dimerization required for productive Stn1 binding and efficient telomere maintenance. Structure 21, 109–120 (2013).
Yu, E. Y., Sun, J., Lei, M. & Lue, N. F. Analyses of Candida Cdc13 orthologues revealed a novel OB fold dimer arrangement, dimerization-assisted DNA binding, and substantial structural differences between Cdc13 and RPA70. Mol. Cell. Biol. 32, 186–198 (2012).
Hang, L. E., Liu, X., Cheung, I., Yang, Y. & Zhao, X. SUMOylation regulates telomere length homeostasis by targeting Cdc13. Nat. Struct. Mol. Biol. 18, 920–926 (2011).
Gao, H., Cervantes, R. B., Mandell, E. K., Otero, J. H. & Lundblad, V. RPA-like proteins mediate yeast telomere function. Nat. Struct. Mol. Biol. 14, 208–214 (2007).
Gelinas, A. D. et al. Telomere capping proteins are structurally related to RPA with an additional telomere-specific domain. Proc. Natl Acad. Sci. USA 106, 19298–19303 (2009).
Sun, J. et al. Stn1–Ten1 is an Rpa2–Rpa3-like complex at telomeres. Genes Dev. 23, 2900–2914 (2009).
Lue, N. F. et al. The telomere capping complex CST has an unusual stoichiometry, makes multipartite interaction with G-tails, and unfolds higher-order G-tail structures. PLoS Genet. 9, e1003145 (2013).
Giraud-Panis, M. J., Teixeira, M. T., Geli, V. & Gilson, E. CST meets shelterin to keep telomeres in check. Mol. Cell 39, 665–676 (2010).
Li, S. et al. Cdk1-dependent phosphorylation of Cdc13 coordinates telomere elongation during cell-cycle progression. Cell 136, 50–61 (2009).
Liu, C. C., Gopalakrishnan, V., Poon, L. F., Yan, T. & Li, S. Cdk1 regulates the temporal recruitment of telomerase and Cdc13-Stn1-Ten1 complex for telomere replication. Mol. Cell. Biol. 34, 57–70 (2014).
Teixeira, M. T., Arneric, M., Sperisen, P. & Lingner, J. Telomere length homeostasis is achieved via a switch between telomerase-extendible and -nonextendible states. Cell 117, 323–335 (2004).
Lei, M., Podell, E. R. & Cech, T. R. Structure of human POT1 bound to telomeric single-stranded DNA provides a model for chromosome end-protection. Nat. Struct. Mol. Biol. 11, 1223–1229 (2004).
Lei, M., Podell, E. R., Baumann, P. & Cech, T. R. DNA self-recognition in the structure of Pot1 bound to telomeric single-stranded DNA. Nature 426, 198–203 (2003).
Horvath, M. P., Schweiker, V. L., Bevilacqua, J. M., Ruggles, J. A. & Schultz, S. C. Crystal structure of the Oxytricha nova telomere end binding protein complexed with single strand DNA. Cell 95, 963–974 (1998).
Garvik, B., Carson, M. & Hartwell, L. Single-stranded DNA arising at telomeres in cdc13 mutants may constitute a specific signal for the RAD9 checkpoint. Mol. Cell. Biol. 15, 6128–6138 (1995).
Mersaoui, S. Y., Bonnell, E. & Wellinger, R. J. Nuclear import of Cdc13 limits chromosomal capping. Nucleic Acids Res. 46, 2975–2989 (2018).
Puglisi, A., Bianchi, A., Lemmens, L., Damay, P. & Shore, D. Distinct roles for yeast Stn1 in telomere capping and telomerase inhibition. EMBO J. 27, 2328–2339 (2008).
Miyagawa, K. et al. SUMOylation regulates telomere length by targeting the shelterin subunit Tpz1(Tpp1) to modulate shelterin–Stn1 interaction in fission yeast. Proc. Natl Acad. Sci. USA 111, 5950–5955 (2014).
Taggart, A. K., Teng, S. C. & Zakian, V. A. Est1p as a cell cycle-regulated activator of telomere–bound telomerase. Science 297, 1023–1026 (2002).
Petreaca, R. C. et al. Chromosome end protection plasticity revealed by Stn1p and Ten1p bypass of Cdc13p. Nat. Cell Biol. 8, 748–755 (2006).
Bochkareva, E., Korolev, S., Lees-Miller, S. P. & Bochkarev, A. Structure of the RPA trimerization core and its role in the multistep DNA-binding mechanism of RPA. EMBO J. 21, 1855–1863 (2002).
Price, C. M. et al. Evolution of CST function in telomere maintenance. Cell Cycle 9, 3157–3165 (2010).
Wan, B. et al. The Tetrahymena telomerase p75–p45–p19 subcomplex is a unique CST complex. Nat. Struct. Mol. Biol. 22, 1023–1026 (2015).
Jiang, J. et al. Structure of telomerase with telomeric DNA. Cell 173, 1179–1190.e13 (2018).
Lim, C. J. et al. The structure of human CST reveals a decameric assembly bound to telomeric DNA. Science 368, 1081–1085 (2020).
Vodenicharov, M. D., Laterreur, N. & Wellinger, R. J. Telomere capping in non-dividing yeast cells requires Yku and Rap1. EMBO J. 29, 3007–3019 (2010).
Vodenicharov, M. D. & Wellinger, R. J. DNA degradation at unprotected telomeres in yeast is regulated by the CDK1 (Cdc28/Clb) cell-cycle kinase. Mol. Cell 24, 127–137 (2006).
Larrivee, M., LeBel, C. & Wellinger, R. J. The generation of proper constitutive G-tails on yeast telomeres is dependent on the MRX complex. Genes Dev. 18, 1391–1396 (2004).
Lue, N. F., Chan, J., Wright, W. E. & Hurwitz, J. The CDC13-STN1-TEN1 complex stimulates Pol ɑ activity by promoting RNA priming and primase-to-polymerase switch. Nat. Commun. 5, 5762 (2014).
Grossi, S., Puglisi, A., Dmitriev, P. V., Lopes, M. & Shore, D. Pol12, the B subunit of DNA polymerase ɑ, functions in both telomere capping and length regulation. Genes Dev. 18, 992–1006 (2004).
Wang, F. et al. The POT1–TPP1 telomere complex is a telomerase processivity factor. Nature 445, 506–510 (2007).
Hellman, L. M. & Fried, M. G. Electrophoretic mobility shift assay (EMSA) for detecting protein–nucleic acid interactions. Nat. Protoc. 2, 1849–1861 (2007).
Minor, W., Cymborowski, M., Otwinowski, Z. & Chruszcz, M. HKL-3000: the integration of data reduction and structure solution—from diffraction images to an initial model in minutes. Acta Crystallogr. D Biol. Crystallogr. 62, 859–866 (2006).
Kabsch, W. Integration, scaling, space-group assignment and post-refinement. Acta Crystallogr. D Biol. Crystallogr. 66, 133–144 (2010).
Adams, P. D. et al. PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr. D Biol. Crystallogr. 66, 213–221 (2010).
Emsley, P., Lohkamp, B., Scott, W. G. & Cowtan, K. Features and development of Coot. Acta Crystallogr. D Biol. Crystallogr. 66, 486–501 (2010).
McCoy, A. J. et al. Phaser crystallographic software. J. Appl. Crystallogr. 40, 658–674 (2007).
Wu, Z. F. et al. Rad6–Bre1-mediated H2B ubiquitination regulates telomere replication by promoting telomere-end resection. Nucleic Acids Res. 45, 3308–3322 (2017).
Wu, Z. et al. Rad6–Bre1 mediated histone H2Bub1 protects uncapped telomeres from exonuclease Exo1 in Saccharomyces cerevisiae. DNA Repair (Amst.) 72, 64–76 (2018).
Wu, J. R. & Gilbert, D. M. Rapid DNA preparation for 2D gel analysis of replication intermediates. Nucleic Acids Res. 23, 3997–3998 (1995).
Booth, C., Griffith, E., Brady, G. & Lydall, D. Quantitative amplification of single-stranded DNA (QAOS) demonstrates that cdc13−1 mutants generate ssDNA in a telomere to centromere direction. Nucleic Acids Res. 29, 4414–4422 (2001).
Kyriakou, D. et al. Functional characterisation of long intergenic non-coding RNAs through genetic interaction profiling in Saccharomyces cerevisiae. BMC Biol. 14, 106 (2016).
Liu, J. et al. Reducing sphingolipid synthesis orchestrates global changes to extend yeast lifespan. Aging Cell 12, 833–841 (2013).
Longtine, M. S. et al. Additional modules for versatile and economical PCR-based gene deletion and modification in Saccharomyces cerevisiae. Yeast 14, 953–961 (1998).
Acknowledgements
We thank the staff members from BL18U1 and BL19U1 beamlines at the National Facility for Protein Science in Shanghai, Zhangjiang Laboratory for help with crystal data collection and the protein production team at Shanghai Institute of Precision Medicine for technical assistance. This work was supported by grants from the Ministry of Science and Technology of China (grant no. 2018YFA0107004 to M.L.), the National Natural Science Foundation of China (grant nos. 31525007 and U1632267 to M.L., grant no. U1732124 to J.W. and grant nos. 21625302 and 21573217 to G.L.), the Outstanding Academic Leader Program of Science and Technology Commission of Shanghai Municipality (grant no. 16XD1405000 to M.L. and grant no. 19XD1422200 to J.W.) and the Shanghai Municipal Education Commission–Gaofeng Clinical Medicine Grant Support (grant no. 20181711 to J.W.).
Author information
Authors and Affiliations
Contributions
M.L. and J.W. conceived this study. Y.G. and Z.W. carried out the bulk of the experiments. Y.G. and S.S. carried out cloning and protein expression. J.W. and Y.G. carried out structure determination and crystallographic analysis and interpreted the results. Z.W. performed the bulk of yeast genetic experiments, including telomere Southern blot, QAOS and all ChIP assays. G.L. and Q.Z. performed the simulation analysis. H.C. and G.L. contributed to the data interpretation and results discussions. M.L., J.W. and Z.W. wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Beth Moorefield was the primary editor on this article and managed its editorial process and peer review in collaboration with the rest of the editorial team.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Structural analyses of the Cdc13OB234-Tel25 interaction.
a, Characterization of the interaction between KlCdc13OB234 and KlStn1 by yeast two-hybrid (Y2H) assay. The direct interaction was determined by measuring the β-galactosidase activity produced by the reporter gene. Data are averages of three independent β-galactosidase measurements normalized to the largest value of the KlStn1OB5^WH-KlCdc13OB234 interaction arbitrarily set to 100. The fragments of KlCdc13, KlStn1 and KlTen1 used in this Y2H assay are shown in the right panel. b, Gel filtration profile of KlCdc13OB234 protein on a Superdex 200 column. The peak of KlCdc13OB234 was resolved by SDS-PAGE and stained with Coomassie brilliant blue. c, Characterization of the interaction between KlCdc13OB234 and single-stranded DNA (ssDNA) by electrophoretic mobility shift assay. The protein KlCdc13OB234 (1 μM) was incubated with 6-FAM labelled telomeric ssDNA (2 nM) and then analyzed by native PAGE. Tel50, Tel38–1, Tel38–2 and Tel25 contain 2 (50 nt), 1.5 (38 nt), 1.5 (38 nt) and 1 (25 nt) telomeric repeats as indicated. d, Electron density map of the Tel25 in the KlCdc13OB234-Tel25 complex. Stereo view of the Sigma-A weighted 2Fo-Fc map shows that Tel25 is ordered in the crystal. Refined model of Tel25 is superimposed on the electron density map. Contours are drawn at the 1.0 σ level. e, An electrostatic potential surface representation of the DBD domain surrounded by Tel25. Positive potential, blue; negative potential, red (at the 10 kT e-1 level). Uncropped images for panels b,c are available as source data.
Extended Data Fig. 2 ITC measurements of the wild-type and mutant Cdc13OB234-Tel25 interactions.
Mutants of Tel25 (a) or Cdc13OB234 (b) interfere with the Cdc13OB234-Tel25 interaction at different degrees.
Extended Data Fig. 3 Structural and mutational analyses of the Cdc13OB2-Cdc13OB4 interaction.
a, Homodimeric structures of ScCdc13OB2 (PDB: 4HCE) and CgCdc13OB4 (PDB: 3RMH). b, Co-IP of Flag-ScCdc13OB2, Myc-ScCdc13OB2 and Myc-ScCdc13OB4 showed that only the interaction between ScCdc13OB2 and ScCdc13OB4 can be detected. The levels of each protein in the input and IP samples were analyzed by immunoblotting with the indicated antibodies. GAPDH was used as a loading control. c, Intramolecular interaction and self-association of CgCdc13OB2 and CgCdc13OB4 were examined in yeast two-hybrid assays. Direct interaction between CgCdc13OB2 and CgCdc13OB4 was determined by measuring the β-galactosidase activity produced by the reporter gene. Data are averages of three independent β-galactosidase measurements normalized to the largest value of the CgCdc13OB2-CgCdc13OB4 interaction arbitrarily set to 100. Error bars in the graph represent indicate mean ± s.e.m. Uncropped images for panel b are available as source data.
Extended Data Fig. 4 Multiple sequence alignment of Cdc13 proteins from various budding yeast species.
Secondary structure elements of ScCdc13 and KlCdc13 are labeled on the top and bottom of the sequences, respectively. Five domains are boxed with respective colors as in Fig. 1a. Conserved residues are boxed and highlighted in red.
Extended Data Fig. 5 Functional analysis of the Cdc13OB2-Cdc13OB4 intramolecular interface.
a, Temperature-dependent effects of the P371S and L401R mutations on ScCdc13OB2 stability in cells. b, Temperature-dependent effects of the P401S and F433R mutations on KlCdc13OB2 stability in cells. c, Co-IP of KlCdc13OB4-Flag and Strep-KlCdc13OB2. The levels of each protein in the whole cell extract and Flag IP samples were analyzed by immunoblotting with the indicated antibodies. The non-specific bands are indicated in red star. d, Co-IP of GST-ScCdc13OB4 and ScCdc13OB2-His. The levels of each protein in the whole cell extract and GST IP samples were analyzed by immunoblotting with the indicated antibodies. e, Y2H assay shows that both Cdc13OB234 and Cdc13OB2^4 efficiently interact with Stn1WH. Data are averages of three independent β-galactosidase measurements normalized to the largest value of the KlStn1OB5^WH-KlCdc13OB234 interaction arbitrarily set to 100. Error bars in the graph indicate mean ± s.e.m. f, g, Y2H assay shows that Cdc13OB2^4, but not Cdc13OB2 and Cdc13OB4 alone, mediates the interaction with Stn1WH in both K. lactis (f) and S. cerevisiae (g). Data are averages of three independent β-galactosidase measurements normalized to the largest value of the KlStn1WH-KlCdc13OB2^4 (f) and the ScStn1WH-ScCdc13OB2^4 (g) interactions arbitrarily set to 100. Error bars indicate mean ± s.e.m.. Uncropped images for panels a-d are available as source data.
Extended Data Fig. 6 Structural and mutational analyses of the Cdc13OB2^4-Stn1WH interaction.
a, Ribbon diagram of the Stn1WH with WH1 colored in yellow and WH2 in blue. Secondary structure elements are labeled. b, Superposition of the KlStn1WH and ScStn1WH (PDB: 3KEY) crystal structures. KlStn1 WH1 and WH2 are colored in yellow and blue and ScStn1 WH1 and WH2 in pink and grey. c, Electrostatic surface potential of the Cdc13OB2^4-Stn1WH complex with a 2:2 stoichiometry (red: negative, blue: positive). d, ITC measurements of WT and mutant Cdc13OB2^4-Stn1WH interactions. e, Y2H analysis of WT and mutant Cdc13OB2^4-Stn1WH interactions. Data are averages of three independent β-galactosidase measurements normalized to the largest value of the KlStn1WH-KlCdc13OB2^4 interaction arbitrarily set to 100. Error bars in the graph indicate mean ± s.e.m.
Extended Data Fig. 7 Sequence alignments of K. lactis and S. cerevisiae Cdc13OB2, Cdc13OB4 and conserved residues of Stn1 at the Cdc13OB2^4-Stn1WH interface.
a-c, Sequence alignment of K. lactis and S. cerevisiae Cdc13OB2 (a), Cdc13OB4 (b) and Stn1 (c), respectively. Secondary structure elements of KlCdc13 and ScCdc13 are labeled on the top and bottom of the sequences, respectively. Conserved residues are boxed and highlighted in red. Red triangles, red stars and blue arrow heads denote residues important for the Cdc13OB2-Cdc13OB4, Cdc13OB2^4-Stn1WH and the Stn1OB5-Ten1 interactions, respectively. d, Y2H analysis shows that mutations of key interacting residues at the interface disrupt the S. cerevisiae Cdc13OB2^4-Stn1WH interaction. Data are averages of three independent β-galactosidase measurements normalized to the largest value of the ScStn1WH-ScCdc13OB2^4 interaction arbitrarily set to 100. Error bars indicate mean ± s.e.m. e, f, Co-IP assays of Strep-ScCdc13 and ScStn1-His (e), and Strep-ScCdc13 and ScEst1-His (f) when overexpressed in yeast cells. The levels of each protein in the input and IP samples were analyzed by immunoblotting with the indicated antibodies. Uncropped images for panels e,f are available as source data.
Extended Data Fig. 8 Structural and Functional analysis of the Cdc13-Stn1 and Stn1-Ten1 interactions.
a, Cdc13E867R mutant protein was expressed at the wild-type level. Flag-tagged Cdc13WT and Cdc13E867R mutant proteins were ectopically expressed under the control of the native Cdc13 promoter. The expression level was verified by quantitative western blot analysis, and the expression levels of tubulin are used as a control. b, Mean expression levels for three independent clones of each strain in (a) are plotted. The error bars indicate mean ± s.e.m. c, Disruption of Cdc13-Stn1 interaction shows no effect on cell viability at all temperature detected. d, EMSA analysis of the interaction between the KlStn1OB5-KlTen1 complex and Tel25. Increasing amounts of the KlStn1OB5-KlTen1 complex (0, 0.9, 1.8, 3.7, 7.5, 15 μM) were incubated with Tel25 and then analyzed by native PAGE. e, Structural comparison of the KlStn1OB5-KlTen1 and the CtStn1OB5-CtTen1 (PDB: 3KF8) complexes. f, Y2H analyses of the wild-type and mutant KlStn1OB5-KlTen1 interaction. Data are averages of three independent β-galactosidase measurements normalized to the largest value of the wild-type KlStn1OB5-KlTen1 interaction arbitrarily set to 100. Error bars indicate mean ± s.e.m. g, Sequence alignment of K. lactis and S. cerevisiae Ten1. Secondary structure elements of KlTen1 are labeled on the top of the sequences. Conserved residues are boxed and highlighted in red. Blue arrow head denotes the arginine residue that forms a conserved salt bridge with Stn1. h, Y2H analyses of the wild-type and mutant ScStn1OB5-ScTen1 interactions, respectively. Data are averages of three independent β-galactosidase measurements normalized to the largest value of the wild-type ScStn1OB5-ScTen1 interaction arbitrarily set to 100. Error bars in the graph indicate mean ± s.e.m. Uncropped images for panels a,c,d are available as source data.
Extended Data Fig. 9 Comparison of the CST and RPA complexes.
a, Architecture comparison of the CST and RPA complexes. The RPA complex structure (PDB: 4GOP) does not contain the OB1 fold. For the purpose of comparison, the OB1 fold in Cdc13 is also not shown in the CST complex. b, The domain organizations of the CST and RPA complexes.
Extended Data Fig. 10 Sequence and functional analyses of C. albicans Cdc13A and Cdc13B.
a, Sequence similarity analyses of CaCdc13A and CaCdc13B with ScCdc13 and KlCdc13. The identities of the amino acid sequences between homologous domains were indicated. b, Y2H results show that CaCdc13OB2 and CaCdc13OB4 stably interact with each other, and this OB2-OB4 module of CaCdc13 is required for its interaction with CaStn1WH. Data are averages of three independent β-galactosidase measurements normalized to the largest value of the CaCdc13BOB2^CaCdc13AOB4-CaStn1WH interaction arbitrarily set to 100. Error bars in the graph indicate mean ± s.e.m.
Supplementary information
Supplementary Information
Supplementary Tables 1–6 and Note 1.
Supplementary Video 1
Representative self-assembly trajectory of the KlCdc13OB2^4–KlStn1WH tetramer. Four protomers (two KlCdc13OB2^4 and two KlStn1WH), initially placed far apart randomly, self-assemble into a stable heterotetramer during Gō simulations. KlCdc13OB2^4 is shown as blue and red ribbons and KlStn1WH as gray and orange ribbons.
Supplementary Video 2
The CST model was built based on the crystal structures of KlCdc13OB234–Tel25, KlCdc13OB2^4–KlStn1WH and KlStn1OB5–KlTen1 reported in this work, as well as that of homodimeric ScCdc13OB1 (PDB 3OIP).
Source data
Source Data Fig. 3
Unprocessed western blots and yeast growth on plates for Fig. 3d,g,h
Source Data Fig. 3
Statistical Source Data for Fig. 3i
Source Data Fig. 4
Unprocessed Southern blots for Fig. 4f–h
Source Data Fig. 4
Statistical Source Data for Fig. 4g
Source Data Fig. 5
Statistical Source Data for Fig. 5a–c
Source Data Fig. 5
Unprocessed Southern blots for Fig. 5e
Source Data Fig. 6
Unprocessed tetrad dissection on plates for Fig. 6c
Source Data Extended Data Fig. 1
Unprocessed SDS–PAGE gel and EMSA gel for Extended Data Fig. 1b,c
Source Data Extended Data Fig. 3
Unprocessed western blots for Extended Data Fig. 3b
Source Data Extended Data Fig. 5
Unprocessed western blots for Extended Data Fig. 5a–d
Source Data Extended Data Fig. 7
Unprocessed western blots for Extended Data Fig. 7e,f
Source Data Extended Data Fig. 8
Unprocessed western blots, yeast growth on plates and EMSA gel for Extended Data Fig. 8a,c,d
Rights and permissions
About this article
Cite this article
Ge, Y., Wu, Z., Chen, H. et al. Structural insights into telomere protection and homeostasis regulation by yeast CST complex. Nat Struct Mol Biol 27, 752–762 (2020). https://doi.org/10.1038/s41594-020-0459-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41594-020-0459-8
This article is cited by
-
Structure of Tetrahymena telomerase-bound CST with polymerase α-primase
Nature (2022)
-
Shaping human telomeres: from shelterin and CST complexes to telomeric chromatin organization
Nature Reviews Molecular Cell Biology (2021)