Abstract
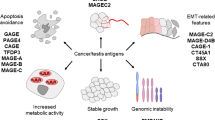
Cancer/testis antigens (CTAs) are a group of tumour-associated antigens (TAAs) that display normal expression in the adult testis—an immune-privileged organ—but aberrant expression in several types of cancers, particularly in advanced cancers with stem cell-like characteristics. There has been an explosion in CTA-based research since CTAs were first identified in 1991 and MAGE-1 was shown to elicit an autologous cytotoxic T-lymphocyte (CTL) response in a patient with melanoma. The resulting data have not only highlighted a role for CTAs in tumorigenesis, but have also underscored the translational potential of these antigens for detecting and treating many types of cancers. Studies that have investigated the use of CTAs for the clinical management of urological malignancies indicate that these TAAs have potential roles as novel biomarkers, with increased specificity and sensitivity compared to those currently used in the clinic, and therapeutic targets for cancer immunotherapy. Increasing evidence supports the utilization of these promising tools for urological indications.
Key Points
-
Restricted expression of cancer/testis antigens (CTAs) in an immune-privileged organ (the adult testis) and aberrant expression in malignant tissue make CTAs ideal biomarkers and immunotherapeutic targets for managing urological cancers
-
Unlike many cancer-associated genes, which frequently harbour mutations associated with their pathological function, mutations in genes encoding the CTAs are extremely rare
-
The majority of CTAs are thought to be intrinsically disordered proteins that often engage in promiscuous interactions with other proteins when overexpressed
-
A key problem for CTA-based therapies is tumour cell heterogeneity, which results in differential expression of CTAs; use of the FDA-approved DNA methylation inhibitor decitabine can circumvent this issue
-
Use of a combination of markers is likely to improve the accuracy of prognostication and treatment stratification compared to utilization of a single marker
-
The success of clinical trials underscores the immunotherapeutic potential of a CTA-based approach and indicates that CTAs could be used as clinical tools for urological malignancies in the near future
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
O'Callaghan, T. Introduction: the prevention agenda. Nature 471, 2–4 (2011).
Jemal, A. et al. Global cancer statistics. CA Cancer J. Clin. 61, 69–90 (2011).
Kinsella, K. & Wan, H. E. International population report. An aging world. (U.S. Government Printing Office, Washington DC, 2009).
Arteaga, C. L. & Baselga, J. Impact of genomics on personalized cancer medicine. Clin. Cancer Res. 18, 612–618 (2012).
Parkinson, D. R., Johnson, B. E. & Sledge, G. W. Making personalized cancer medicine a reality: challenges and opportunities in the development of biomarkers and companion diagnostics. Clin. Cancer Res. 18, 619–624 (2012).
Tannock, I. F. et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N. Engl. J. Med. 351, 1502–1512 (2004).
Aragon-Ching, J. B. & Dahut, W. L. Chemotherapy in androgen-independent prostate cancer (AIPC): what's next after taxane progression? Cancer Ther. 5, 151–160 (2007).
Foley, R., Marignol, L., Keane, J. P., Lynch, T. H. & Hollywood, D. Androgen hypersensitivity in prostate cancer: molecular perspectives on androgen deprivation therapy strategies. Prostate 71, 550–557 (2011).
Zachos, I. et al. Systemic therapy of metastatic bladder cancer in the molecular era: current status and future promise. Expert Opin. Investig. Drugs 19, 875–887 (2010).
Scanlan, M. J. et al. Identification of cancer/testis genes by database mining and mRNA expression analysis. Int. J. Cancer 98, 485–492 (2002).
Simpson, A. J., Caballero, O. L., Jungbluth, A., Chen, Y. T. & Old, L. J. Cancer/testis antigens, gametogenesis and cancer. Nat. Rev. Cancer 5, 615–625 (2005).
Bera, T. K. et al. Selective POTE paralogs on chromosome 2 are expressed in human embryonic stem cells. Stem Cells Dev. 17, 325–332 (2008).
Gjerstorff, M. F. et al. Distinct GAGE and MAGE-A expression during early human development indicate specific roles in lineage differentiation. Hum. Reprod. 23, 2194–2201 (2008).
Lifantseva, N. et al. Expression patterns of cancer-testis antigens in human embryonic stem cells and their cell derivatives indicate lineage tracks. Stem Cells Int. http://dx.doi:10.4061/2011/795239.
Caballero, O. L. et al. Frequent MAGE mutations in human melanoma. PLoS ONE 5, e12773 (2010).
Clark, J. et al. Identification of novel genes, SYT and SSX, involved in the t(X;18)(p11.2;q11.2) translocation found in human synovial sarcoma. Nat. Genet. 7, 502–508 (1994).
Cerveira, N. et al. A novel spliced fusion of MLL with CT45A2 in a pediatric biphenotypic acute leukemia. BMC Cancer 10, 518 (2010).
Sigalotti, L. et al. Promoter methylation controls the expression of MAGE2, 3 and 4 genes in human cutaneous melanoma. J. Immunother. 25, 16–26 (2002).
Sigalotti, L. et al. Intratumor heterogeneity of cancer/testis antigens expression in human cutaneous melanoma is methylation-regulated and functionally reverted by 5-aza-2'-deoxycytidine. Cancer Res. 64, 9167–9171 (2004).
Karpf, A. R. A potential role for epigenetic modulatory drugs in the enhancement of cancer/germ-line antigen vaccine efficacy. Epigenetics 1, 116–120 (2006).
Akers, S. N., Odunsi, K. & Karpf, A. R. Regulation of cancer germline antigen gene expression: implications for cancer immunotherapy. Future Oncol. 6, 717–732 (2010).
Yegnasubramanian, S. et al. DNA hypomethylation arises later in prostate cancer progression than CpG island hypermethylation and contributes to metastatic tumor heterogeneity. Cancer Res. 68, 8954–8967 (2008).
Glazer, C. A. et al. Integrative discovery of epigenetically derepressed cancer testis antigens in NSCLC. PLoS ONE 4, e8189 (2009).
Suyama, T. et al. Expression of cancer/testis antigens in prostate cancer is associated with disease progression. Prostate 70, 1778–1787 (2010).
Janic, A., Mendizabal, L., Llamazares, S., Rossell, D. & Gonzalez, C. Ectopic expression of germline genes drives malignant brain tumor growth in Drosophila. Science 330, 1824–1827 (2010).
Scanlan, M. J., Simpson, A. J. & Old, L. J. The cancer/testis genes: review, standardization, and commentary. Cancer Immun. 4, 1 (2004).
Postovit, L. M. et al. The commonality of plasticity underlying multipotent tumor cells and embryonic stem cells. J. Cell Biochem. 101, 908–917 (2007).
Ben-Porath, I. et al. An embryonic stem cell-like gene expression signature in poorly differentiated aggressive human tumors. Nat. Genet. 40, 499–507 (2008).
Kim, J. et al. A Myc network accounts for similarities between embryonic stem and cancer cell transcription programs. Cell 143, 313–324 (2010).
Ma, Y. L. et al. Human embryonic stem cells and metastatic colorectal cancer cells shared the common endogenous human microRNA-26b. J. Cell Mol. Med. 15, 1941–1954 (2011).
Stevenson, B. J. et al. Rapid evolution of cancer/testis genes on the X chromosome. BMC Genomics 8, 129 (2007).
Caballero, O. L. & Chen, Y. T. Cancer/testis (CT) antigens: potential targets for immunotherapy. Cancer Sci. 100, 2014–2021 (2009).
Scanlan, M. J., Gure, A. O., Jungbluth, A. A., Old, L. J. & Chen, Y. T. Cancer/testis antigens: an expanding family of targets for cancer immunotherapy. Immunol. Rev. 188, 22–32 (2002).
Almeida, L. G. et al. CTdatabase: a knowledge-base of high-throughput and curated data on cancer-testis antigens. Nucleic Acids Res. 37, 816–819 (2009).
Johns Hopkins University School of Medicine. ACTAbase: a comprehensive database for cancer/testis antigens [online], (2011).
Andrade, V. C. et al. Prognostic impact of cancer/testis antigen expression in advanced stage multiple myeloma patients. Cancer Immun. 8, 2 (2008).
Schroder, F. H. et al. Screening and prostate-cancer mortality in a randomized European study. N. Engl. J. Med. 360, 1320–1328 (2009).
Andriole, G. L. et al. Mortality results from a randomized prostate-cancer screening trial. N. Engl. J. Med. 360, 1310–1319 (2009).
Draisma, G. et al. Lead time and overdiagnosis in prostate-specific antigen screening: importance of methods and context. J. Natl Cancer Inst. 101, 374–383 (2009).
Fossa, A. et al. NY-ESO-1 protein expression and humoral immune responses in prostate cancer. Prostate 59, 440–447 (2004).
Hudolin, T. et al. Immunohistochemical expression of tumor antigens MAGE-A1, MAGE-A3/4, and NY-ESO-1 in cancerous and benign prostatic tissue. Prostate 66, 13–18 (2006).
Smith, H. A., Cronk, R. J., Lang, J. M. & McNeel, D. G. Expression and immunotherapeutic targeting of the SSX family of cancer-testis antigens in prostate cancer. Cancer Res. 71, 6785–6795 (2011).
Shiraishi, T. et al. Cancer/testis antigens as potential predictors of biochemical recurrence of prostate cancer following radical prostatectomy. J. Transl. Med. 9, 153 (2011).
Xie, C. et al. A novel multiplex assay combining autoantibodies plus PSA has potential implications for classification of prostate cancer from non-malignant cases. J. Transl. Med. 9, 43 (2011).
Szmania, S., Tricot, G. & van Rhee, F. NY-ESO-1 immunotherapy for multiple myeloma. Leuk. Lymphoma 47, 2037–2048 (2006).
Tyagi, P. & Mirakhur, B. MAGRIT: the largest-ever phase III lung cancer trial aims to establish a novel tumor-specific approach to therapy. Clin. Lung Cancer 10, 371–374 (2009).
Nicholaou, T. et al. Regulatory T-cell-mediated attenuation of T-cell responses to the NY-ESO-1 ISCOMATRIX vaccine in patients with advanced malignant melanoma. Clin. Cancer Res. 15, 2166–2173 (2009).
US National Library of Medicine. ClinicalTrials.gov [online], (2011).
Dubovsky, J. A. & McNeel, D. G. Inducible expression of a prostate cancer-testis antigen, SSX-2, following treatment with a DNA methylation inhibitor. Prostate 67, 1781–1790 (2007).
Smith, H. A. & McNeel, D. G. Vaccines targeting the cancer-testis antigen SSX-2 elicit HLA-A2 epitope-specific cytolytic T cells. J. Immunother. 34, 569–580 (2011).
Yokokawa, J. et al. Identification of cytotoxic T-lymphocyte epitope(s) and its agonist epitope(s) of a novel target for vaccine therapy (PAGE4). Int. J. Cancer 121, 595–605 (2007).
Parmigiani, R. B. et al. Characterization of a cancer/testis (CT) antigen gene family capable of eliciting humoral response in cancer patients. Proc. Natl Acad. Sci. USA 103, 18066–18071 (2006).
Parmigiani, R. B. et al. Antibodies against the cancer-testis antigen CTSP-1 are frequently found in prostate cancer patients and are an independent prognostic factor for biochemical-recurrence. Int. J. Cancer 122, 2385–2390 (2008).
Miles, A. K. et al. Identification of a novel prostate cancer-associated tumor antigen. Prostate 67, 274–287 (2007).
Chiriva-Internati, M. et al. Identification of AKAP-4 as a new cancer/testis antigen for detection and immunotherapy of prostate cancer. Prostate 72, 12–23 (2012).
Fong, L. et al. Potentiating endogenous antitumor immunity to prostate cancer through combination immunotherapy with CTLA4 blockade and GM-CSF. Cancer Res. 69, 609–615 (2009).
Karpf, A. R., Bai, S., James, S. R., Mohler, J. L. & Wilson, E. M. Increased expression of androgen receptor coregulator MAGE-11 in prostate cancer by DNA hypomethylation and cyclic AMP. Mol. Cancer Res. 7, 523–535 (2009).
Bai, S., He, B. & Wilson, E. M. Melanoma antigen gene protein MAGE-11 regulates androgen receptor function by modulating the interdomain interaction. Mol. Cell Biol. 25, 1238–1257 (2005).
Wilson, E. M. Androgen receptor molecular biology and potential targets in prostate cancer. Ther. Adv. Urol. 2, 105–117 (2010).
Cronwright, G. et al. Cancer/testis antigen expression in human mesenchymal stem cells: down-regulation of SSX impairs cell migration and matrix metalloproteinase 2 expression. Cancer Res. 65, 2207–2215 (2005).
Xiang, Y. et al. JARID1B is a histone H3 lysine 4 demethylase up-regulated in prostate cancer. Proc. Natl Acad. Sci. USA 104, 19226–19231 (2007).
Kaufman, D. S., Shipley, W. U. & Feldman, A. S. Bladder cancer. Lancet 374, 239–249 (2009).
Tanaka, M. F. & Sonpavde, G. Diagnosis and management of urothelial carcinoma of the bladder. Postgrad. Med. 123, 43–55 (2011).
Babjuk, M. et al. EAU guidelines on non-muscle-invasive urothelial carcinoma of the bladder. Eur. Urol. 54, 303–314 (2008).
Herr, H. W. et al. Neoadjuvant chemotherapy in invasive bladder cancer: the evolving role of surgery. J. Urol. 144, 1083–1088 (1990).
Patard, J. J. et al. Expression of MAGE genes in transitional-cell carcinomas of the urinary bladder. Int. J. Cancer 64, 60–64 (1995).
Jungbluth, A. A. et al. Expression of MAGE-antigens in normal tissues and cancer. Int. J. Cancer 85, 460–465 (2000).
Bergeron, A. et al. High frequency of MAGE-A4 and MAGE-A9 expression in high-risk bladder cancer. Int. J. Cancer 125, 1365–1371 (2009).
Schultz-Thater, E. et al. MAGE-A10 is a nuclear protein frequently expressed in high percentages of tumor cells in lung, skin and urothelial malignancies. Int. J. Cancer 129, 1137–1148 (2011).
Chen, Y. T. et al. A testicular antigen aberrantly expressed in human cancers detected by autologous antibody screening. Proc. Natl Acad. Sci. USA 94, 1914–1918 (1997).
Bolli, M. et al. NY-ESO-1/LAGE-1 coexpression with MAGE-A cancer/testis antigens: a tissue microarray study. Int. J. Cancer 115, 960–966 (2005).
Ono, T. et al. Identification of proacrosin binding protein sp32 precursor as a human cancer/testis antigen. Proc. Natl Acad. Sci. USA 98, 3282–3287 (2001).
Matsuda, R. et al. LY6K is a novel molecular target in bladder cancer on basis of integrate genome-wide profiling. Br. J. Cancer 104, 376–386 (2011).
Hayami, S. et al. Overexpression of the JmjC histone demethylase KDM5B in human carcinogenesis: involvement in the proliferation of cancer cells through the E2F/RB pathway. Mol. Cancer 9, 59 (2010).
Nishiyama, T. et al. Immunotherapy of bladder cancer using autologous dendritic cells pulsed with human lymphocyte antigen-A24-specific MAGE-3 peptide. Clin. Cancer Res. 7, 23–31 (2001).
Picard, V., Bergeron, A., Larue, H. & Fradet, Y. MAGE-A9 mRNA and protein expression in bladder cancer. Int. J. Cancer 120, 2170–2177 (2007).
Parkin, C. M., Whelan, S. L., Ferlay, J., Teppo, L. & Thomas, D. IARC Scientific Publications No. 155. (International Agency for Research on Cancer, Lyon, France, 2002).
National Cancer Institute. Surveillance Research Program; Cancer Statistics Branch. SEER Program Public Use Data Tapes 1973–2002 (2005).
Motzer, R. J., Bander, N. H. & Nanus, D. M. Renal-cell carcinoma. N. Engl. J. Med. 335, 865–875 (1996).
Karakiewicz, P. I. et al. Multi-institutional validation of a new renal cancer-specific survival nomogram. J. Clin. Oncol. 25, 1316–1322 (2007).
Lam, J. S., Leppert, J. T., Figlin, R. A. & Belldegrun, A. S. Role of molecular markers in the diagnosis and therapy of renal cell carcinoma. Urology 66, 1–9 (2005).
Zini, L. et al. Population-based assessment of survival after cytoreductive nephrectomy versus no surgery in patients with metastatic renal cell carcinoma. Urology 73, 342–346 (2009).
Yagoda, A. Phase II cytotoxic chemotherapy trials in renal cell carcinoma: 1983–1988. Prog. Clin. Biol. Res. 350, 227–241 (1990).
Motzer, R. J. & Russo, P. Systemic therapy for renal cell carcinoma. J. Urol. 163, 408–417 (2000).
Jungbluth, A. A. et al. Immunohistochemical analysis of NY-ESO-1 antigen expression in normal and malignant human tissues. Int. J. Cancer 92, 856–860 (2001).
Yamanaka, K. et al. Expression of MAGE genes in renal cell carcinoma. Int. J. Mol. Med. 2, 57–60 (1998).
Garg, M. et al. Sperm-associated antigen 9 is associated with tumor growth, migration, and invasion in renal cell carcinoma. Cancer Res. 68, 8240–8248 (2008).
Bukowski, R. M. Immunotherapy in renal cell carcinoma. Oncology (Williston Park) 13, 801–813 (1999).
Schmidinger, M. et al. Sequential administration of interferon gamma and interleukin-2 in metastatic renal cell carcinoma: results of a phase II trial. Austrian Renal Cell Carcinoma Study Group. Cancer Immunol. Immunother. 49, 395–400 (2000).
Coral, S. et al. 5-aza-2'-deoxycytidine-induced expression of functional cancer testis antigens in human renal cell carcinoma: immunotherapeutic implications. Clin. Cancer Res. 8, 2690–2695 (2002).
Siegel, R., Naishadham, D. & Jemal, A. Cancer statistics, 2012. CA Cancer J. Clin. 62, 10–29 (2012).
Rosen, A., Jayram, G., Drazer, M. & Eggener, S. E. Global trends in testicular cancer incidence and mortality. Eur. Urol. 60, 374–379 (2011).
Feldman, D. R., Bosl, G. J., Sheinfeld, J. & Motzer, R. J. Medical treatment of advanced testicular cancer. JAMA 299, 672–684 (2008).
Oosterhuis, J. W. & Looijenga, L. H. Testicular germ-cell tumours in a broader perspective. Nat. Rev. Cancer 5, 210–222 (2005).
Jacobsen, G. K. Alpha-fetoprotein (AFP) and human chorionic gonadotropin (HCG) in testicular germ cell tumours. A comparison of histologic and serologic occurrence of tumour markers. Acta Pathol. Microbiol. Immunol. Scand. A. 91, 183–190 (1983).
Rajpert-De Meyts, E. Developmental model for the pathogenesis of testicular carcinoma in situ: genetic and environmental aspects. Hum. Reprod. Update 12, 303–323 (2006).
Okita, K., Ichisaka, T. & Yamanaka, S. Generation of germline-competent induced pluripotent stem cells. Nature 448, 313–317 (2007).
Geldmacher, A., Freier, A., Losch, F. O. & Walden, P. Therapeutic vaccination for cancer immunotherapy: antigen selection and clinical responses. Hum. Vaccin. 7, S115–S119 (2011).
Fratta, E. et al. The biology of cancer testis antigens: putative function, regulation and therapeutic potential. Mol. Oncol. 5, 164–182 (2011).
Garcia-Manero, G. Treatment of higher-risk myelodysplastic syndrome. Semin. Oncol. 38, 673–681 (2011).
Coral, S. et al. Prolonged upregulation of the expression of HLA class I antigens and costimulatory molecules on melanoma cells treated with 5-aza-2'-deoxycytidine (5-AZA-CdR). J. Immunother. 22, 16–24 (1999).
Penney, K. L. et al. mRNA expression signature of Gleason grade predicts lethal prostate cancer. J. Clin. Oncol. 29, 2391–2396 (2011).
Cuzick, J. et al. Prognostic value of an RNA expression signature derived from cell cycle proliferation genes in patients with prostate cancer: a retrospective study. Lancet Oncol. 12, 245–255 (2011).
Kulkarni, M. M. Chapter 25: digital multiplexed gene expression analysis using the NanoString nCounter system. (Greene Pub. Associates, Brooklyn, NY, 2011).
Cheung, I. Y. & Cheung, N. K. Molecular detection of GAGE expression in peripheral blood and bone marrow: utility as a tumor marker for neuroblastoma. Clin. Cancer Res. 3, 821–826 (1997).
Lu, Y., Wu, L. Q., Lu, Z. H., Wang, X. J. & Yang, J. Y. Expression of SSX-1 and NY-ESO-1 mRNA in tumor tissues and its corresponding peripheral blood expression in patients with hepatocellular carcinoma. Chin. Med. J. (Engl.) 120, 1042–1046 (2007).
de Bono, J. S. et al. Circulating tumor cells predict survival benefit from treatment in metastatic castration-resistant prostate cancer. Clin. Cancer Res. 14, 6302–6309 (2008).
Rajagopalan, K., Mooney, S. M., Parekh, N., Getzenberg, R. H. & Kulkarni, P. A majority of the cancer/testis antigens are intrinsically disordered proteins. J. Cell. Biochem. 112, 3256–3267 (2011).
Vavouri, T., Semple, J. I., Garcia-Verdugo, R. & Lehner, B. Intrinsic protein disorder and interaction promiscuity are widely associated with dosage sensitivity. Cell 138, 198–208 (2009).
Edgar, R., Domrachev, M. & Lash, A. E. Gene Expression Omnibus: NCBI gene expression and hybridization array data repository. Nucleic Acids Res. 30, 207–210 (2002).
University of Auckland. R: a language and environment for statistical computing [online], (2010).
Gentleman, R. C. et al. Bioconductor: open software development for computational biology and bioinformatics. Genome Biol. 5, 80 (2004).
University of Rochester Medical Center. The Comprehensive R Archive Network [online].
Acknowledgements
This work was supported by a National Cancer Institute Specialized Program of Research Excellence, the Bernard L. Schwartz Scholar Award by the Patrick C. Walsh Cancer Research Fund, and the Patana Fund of the Brady Urological Institute.
Author information
Authors and Affiliations
Contributions
All authors contributed equally to researching the article and discussions of content, as well as the writing and editing of the manuscript prior to submission.
Corresponding author
Ethics declarations
Competing interests
A provisional patent application covering an invention on a CTA-based gene signature for prostate cancer has been filed by Johns Hopkins University on behalf of P. Kulkarni, T. Shiraishi and R. H. Getzenberg.
Supplementary information
Supplementary Materials
Cancer/testis antigens in testicular germ cell tumours. (DOC 26 kb)
Supplementary Table 1
(DOC 202 kb)
Rights and permissions
About this article
Cite this article
Kulkarni, P., Shiraishi, T., Rajagopalan, K. et al. Cancer/testis antigens and urological malignancies. Nat Rev Urol 9, 386–396 (2012). https://doi.org/10.1038/nrurol.2012.117
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrurol.2012.117
This article is cited by
-
Identification and validation of the high expression of pseudogene TCAM1P in cervical cancer via integrated bioinformatics analysis
Cancer Cell International (2022)
-
A Novel Multiepitope Vaccine Against Bladder Cancer Based on CTL and HTL Epitopes for Induction of Strong Immune Using Immunoinformatics Approaches
International Journal of Peptide Research and Therapeutics (2022)
-
Genome-wide association study of prostate-specific antigen levels identifies novel loci independent of prostate cancer
Nature Communications (2017)
-
Specific autoantigens identified by sera obtained from mice that are immunized with testicular germ cells alone
Scientific Reports (2016)
-
The cancer-retina antigen recoverin as a potential biomarker for renal tumors
Tumor Biology (2016)