Abstract
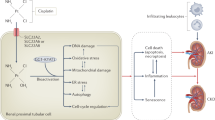
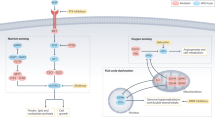
Exposure to potential carcinogens is an etiologic factor for renal cell carcinoma (RCC) and transitional cell carcinoma (TCC) of the urinary bladder. Cytosolic glutathione S-transferases (GSTs) are a superfamily of enzymes that protect normal cells by catalyzing conjugation reactions of electrophilic compounds, including carcinogens, to glutathione. Some GST enzymes possess antioxidant activity against hydroperoxides. The most well characterized classes have been named alpha (GSTA), mu (GSTM), pi (GSTP) and theta (GSTT); each of these classes contains several different isoenzymes. Several types of allelic variation have been identified within classes, with GSTM1-null, GSTT1-null and GSTP1-Ile105/Ile105 conferring impaired catalytic activity. The effects of GSTM1 and GSTT1 polymorphism on susceptibility to RCC depend on exposure to specific chemicals. Individuals with the GSTM1-null genotype carry a higher risk for TCC. The roles of GSTT1 polymorphism in TCC and GSTP1 polymorphisms in both cancers are still controversial. During kidney cancerization, expression of GSTA isoenzymes tends to decrease, which promotes the pro-oxidant environment necessary for RCC growth. In the malignant phenotype of TCC of the bladder, upregulation of various GST classes occurs. Upregulation of GSTT1 and GSTP1 might have important consequences for TCC growth by providing a reduced cellular environment and inhibition of apoptotic pathways.
Key Points
-
Individuals with GSTM1-positive and GSTT1-positive genotypes are at increased risk for renal cell carcinoma (RCC) when occupationally exposed to certain toxicants
-
Individuals with the GSTM1-null genotype are at increased risk for bladder cancer, which might be potentiated by smoking and occupational hazards
-
Data on the influence of GSTT1 polymorphism on the risk for transitional cell carcinoma (TCC) are inconsistent
-
In the process of kidney cancerization, expression of GSTA isoenzymes is downregulated, which contributes to decreased antioxidant capacity in RCC and might enhance RCC growth
-
In the malignant phenotype of TCC of the urinary bladder, upregulation of GSTT1 and GSTP1 might provide a reduced cellular environment and inhibit apoptotic pathways, respectively.
-
GSTP1 enzyme activities are generally retained in RCC, and might influence resistance to chemotherapy; in TCC, GSTP1 should be considered as a potential therapeutic target
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Moore, L. E., Wilson, R. T. & Campleman, S. L. Lifestyle factors, exposures, genetic susceptibility, and renal cell cancer risk: a review. Cancer Invest. 23, 240–255 (2005).
Their, R., Golka, K., Bruning, T., Ko, Y. & Bolt, H. M. Genetic susceptibility to environmental toxicants: the interface between human and experimental studies in the development of new toxicological concepts. Toxicol. Lett. 127, 321–327 (2002).
Hayes, J. D. & Strange, R. C. Glutathione S-transferase polymorphisms and their biological consequences. Pharmacology 61, 154–166 (2000).
Gate, L. & Tew, K. D. Glutathione S-transferases as emerging therapeutic targets. Expert Opin. Ther. Targets 5, 477–489 (2001).
Adler, V. & Pincus, M. R. Effector peptides from glutathione S-transferase-pi affect the activation of jun by jun-N-terminal kinase. Ann. Clin. Lab. Sci. 34, 35–46 (2004).
Habig, W. H., Pabst, M. J. & Jakoby, W. B. Glutathione S-transferase-the first enzymatic step in mercapturic acid formation. J. Biol. Chem. 249, 7130–7139 (1974).
Seidegard, J., Vorachek, W. R., Pero, R. W. & Pearson, W. R. Hereditary difference in the expression of the human glutathione S-transferase active on trans-stilbene oxide are due to a gene deletion. Proc. Natl Acad. Sci. USA 85, 7293–7297 (1988).
Sweeney, C. et al. Glutathione S-transferase M1, T1, and P1 polymorphisms as risk factors for renal cell carcinoma: a case-control study. Cancer Epidemiol. Biomarkers Prev. 9, 449–454 (2000).
Pemble, S. et al. Human glutathione S-transferase theta (GSTT1): cDNA cloning and the characterization of a genetic polymorphism. Biochem. J. 300, 271–276 (1994).
Wiencke, J. K. Pemble, S., Ketterer, B. & Kelsey, K. T. Gene deletion of glutathione S-transferase theta: correlation with induced genetic damage and potential role in endogenous mutagenesis. Cancer Epidemiol. Biomarkers Prev. 4, 253–259 (1995).
Coles, F. B. & Kadlubar, F. F. Human alpha class glutathione S-transferases: genetic polymorphism, expression, and susceptibility to disease. Methods Enzymol. 401, 9–42 (2005).
Simic, T., Pljesa-Ercegovac, M., Savic-Radojevic, A., Hadziahmetovic, M. & Mimic-Oka, J. Identification of a glutathione S-transferase without affinity for glutathione sepharose in human kidney. Amino Acids 30, 495–498 (2006).
Hurst, R., Bao, Y., Jemth, P., Mannervik, B. & Williamson, G. Phospholipid hydroperoxide glutathione peroxidase activity of human glutathione transferases. Biochem. J. 332, 97–100 (1998).
Peters, M. M, Rivera, M. I., Jones, T. W., Monks, T. J. & Lau, S. S. Glutathione conjugates of tert-butyl-hydroquinone, a metabolite of the urinary tract tumour promoter 3-tert-butyl-hydroxyanisole, are toxic to kidney and bladder. Cancer Res. 56, 1006–1011 (1996).
Brüning, T. et al. Glutathione transferase alpha as a marker for tubular damage after trichloroethylene exposure. Arch. Toxicol. 73, 246–254 (1999).
Kellen, E. et al. Pooled analysis and meta-analysis of the glutathione S-transferase P1 Ile105Val polymorphism and bladder cancer: a HuGE-GSEC review. Am. J. Epidemiol. 165, 1221–1230 (2007).
Garte, S. et al. Metabolic gene polymorphism frequencies in control populations. Cancer Epidemiol. Biomarkers Prev. 10, 1239–1248 (2001).
Green, T., Dow, J., Ellis, M. K, Foster, J. R. & Odum, J. The role of glutathione conjugation in the development of kidney tumours in rats exposed to trichloroethylene. Chem. Biol. Interact. 105, 99–117 (1997).
Rodilla, V. et al. Glutathione S-transferases in human renal cortex and neoplastic tissue: enzymatic activity, isoenzyme profile and immunohistochemical localization. Xenobiotica 28, 443–456 (1998).
Simic, T., Mimic-Oka, J., Ille, K., Savic-Radojevic, A. & Reljic, Z. Isoenzyme profile of glutathione S-transferases in human kidney. Urol. Res. 29, 38–44 (2001).
Delbanco, E. H. et al. Glutathione transferase activities in renal carcinomas and adjacent normal renal tissues: factors influencing renal carcinogenesis induced by xenobiotics. Arch. Toxicol. 74, 688–694 (2001).
Singh, S. V., Roberts, B., Gudi, V. A., Ruiz, P. & Awasthi, Y. C. Immunohistochemical localization, purification, and characterization of human urinary bladder glutathione S-transferases. Biochim. Biophys. Acta 1074, 363–370 (1991).
Berendsen, C. L. et al. Glutathione S-transferase activity and subunit composition in transitional cell cancer and mucosa of the human bladder. Urology 49, 644–651 (1997).
Kempkes, M., Golka, K., Reich, S., Reckwitz, T. & Bolt, H. M. Glutathione S-transferase GSTM1 and GSTT1 null genotypes as potential risk factors for urothelial cancer of the bladder. Arch. Toxicol. 71, 123–126 (1996).
Katoh, T. et al. Effects of glutathione S-transferase (GST) M1 and GSTT1 genotypes on urothelial cancer risk. Cancer Lett. 132, 147–152 (1998).
Martin, G. M. et al. Somatic mutations are frequent and increase with age in human kidney epithelial cells. Hum. Mol. Genet. 5, 215–221 (1996).
Longuemaux, S. et al. Candidate genetic modifiers of individual susceptibility to renal cell carcinoma: a study of polymorphic human xenobiotic-metabolizing enzymes. Cancer Res. 59, 2903–2908 (1999).
Buzio, L. et al. Glutathione S-transferases M1–1 and T1–1 as risk modifiers for renal cell cancer associated with occupational exposure to chemicals. Occup. Environ. Med. 60, 789–793 (2003).
Karami, S. et al. Renal cell carcinoma, occupational pesticide exposure and modification by glutathione S-transferase polymorphisms. Carcinogenesis 29, 1567–1571 (2008).
Brüning, T. et al. Influence of polymorphisms of GSTM1 and GSTT1 for risk of renal cell cancer in workers with long-term high occupational exposure to trichloroethene. Arch. Toxicol. 71, 596–599 (1997).
Wiesenhütter, B., Selinski, S., Golka, K., Brüning, T. & Bolt, H. M. Re-assessment of the influence of polymorphisms of phase-II metabolic enzymes on renal cell cancer risk of trichloroethylene-exposed workers. Arch. Occup. Environ. Health 81, 247–251 (2007).
Landi, S. Mammalian class Theta GST and differential susceptibility to carcinogens: a review. Mut. Res. 463, 247–283 (2000).
Golka, K. et al. The influence of polymorphisms of glutathione S-transferases M1 and M3 on the development of human urothelial cancer. J. Toxicol. Environ. Health Part A 71, 881–886 (2008).
Shao, J. et al. Genetic variants of the cytochrome P450 and glutathione S-transferase associated with risk of bladder cancer in a southeastern Chinese population. Int. J. Urol. 15, 216–221 (2008).
Engel, L. S. et al. Pooled analysis and meta-analysis of glutathione S-transferase M1 and bladder cancer: a HuGE review. Am. J. Epidemiol. 56, 95–109 (2002).
Tsukino, H. et al. Glutathione S-transferase (GST) M1, T1 and N-acetyltransferase 2 (NAT2) polymorphisms and urothelial cancer risk with tobacco smoking. Eur. J. Cancer Prev. 13, 509–514 (2004).
García-Closas, M. et al. NAT2 slow acetylation, GSTM1 null genotype, and risk of bladder cancer: results from the Spanish Bladder Cancer Study and meta-analyses. Lancet 366, 649–659 (2005).
Golka, K. et al. Occupational and non-occupational risk factors in bladder cancer patients in an industrialized area located in former East Germany. Aktuelle Urol. 36, 417–422 (2005).
Filiadis, I. & Hrouda, D. Genetic factors in chemically induced transitional cell bladder cancer. BJU Int. 86, 794–801 (2000).
Brockmöller, J., Cascorbi, I., Kerb, R. & Roots, I. Combined analysis of inherited polymorphisms in arylamine N-acetyltransferase 2, glutathione S-transferases M1 and T1, microsomal epoxide hydrolase, and cytochrome P450 enzymes as modulators of bladder cancer risk. Cancer Res. 56, 3915–3925 (1996).
Kim, W. J. et al. GSTT1-null genotype is a protective factor against bladder cancer. Urology 60, 913–918 (2002).
Simic, T. et al. Glutathione S-transferase T1–1 activity upregulated in transitional cell carcinoma of urinary bladder. Urology 65, 1035–1040 (2005).
Harries, L. W., Stubbins, M. J., Forman, D., Howard, G. C. & Wolf, C. R. Identification of genetic polymorphisms at the glutathione S-transferase Pi locus and association with susceptibility to bladder, testicular and prostate cancer. Carcinogenesis 18, 641–644 (1997).
Cao, W. et al. Tobacco smoking, GSTP1 polymorphism, and bladder carcinoma. Cancer 104, 2400–2408 (2005).
Mittal, R. D., Srivastava, D. S., Srivastava, A. M. & Srivastava, B. M. Genetic polymorphism of drug metabolizing enzymes (CYP2E1, GSTP1) and susceptibility to bladder cancer in North India. Asian Pac. J. Cancer Prev. 6, 6–9 (2005).
Salinas, A. E. & Wong, M. G. Glutathione S-transferases—a review. Curr. Med. Chem. 6, 279–309 (1999).
Pljesa-Ercegovac, M. et al. Altered antioxidant capacity in human renal cell carcinoma: role of glutathione-associated enzymes. Urol. Oncol. 26, 175–181 (2008).
Towsend, D. & Tew, K. The role of glutathione S-transferase in anti-cancer drug resistance. Oncogene 22, 7369–7375 (2003).
Turella, P. et al. Proapoptotic activity of new glutathione S-transferase inhibitors. Cancer Res. 65, 3751–3761 (2005).
Henderson, J. C. & Wolf, C. R. Disruption of the glutathione transferase pi class genes. Methods Enzymol. 401, 116–135 (2005).
Lusini, L. et al. Altered glutathione anti-oxidant metabolism during tumor progression in human renal cell carcinoma. Int. J. Cancer 91, 55–59 (2001).
Di Illio. C., Del Boccio, G., Aceto, A. & Fererici, G. Alteration of glutathione transferase isoenzyme concentration in human renal carcinoma. Carcinogenesis 8, 861–864 (1987).
Klone, A. et al. Decreased expression of the glutathione S-transferase alpha and pi genes in human renal cell carcinoma. Carcinogenesis 11, 2179–2183 (1990).
Howie, A. F. et al. Glutathione S-transferase and glutathione peroxidase expression in normal and tumour human tissues. Carcinogenesis 11, 451–458 (1990).
Simic, T., Mimic-Oka, J., Ille, K., Dragicevic, D. & Savic-Radojevic, A. Glutathione S-transferase isoenzyme profile in non-tumor and tumor human kidney tissue. World J. Urol. 20, 385–391 (2003).
Chuang, S. T. et al. Overexpression of glutathione S-transferase alpha in clear cell renal cell carcinoma. Am. J. Clin. Pathol. 123, 421–429 (2005).
Liu, L. et al. Immunohistochemical analysis of chromophobe renal cell carcinoma, renal oncocytoma, and clear cell carcinoma: an optimal and practical panel for differential diagnosis. Arch. Pathol. Lab. Med. 131, 1290–1297 (2007).
Takahashi, M. Molecular subclassification of kidney tumors and the discovery of new diagnostic markers. Oncogene 22, 6810–6818 (2003).
Zhao, T. et al. The role of human glutathione S-transferases hGSTA1–1 and hGSTA2–2 in protection against oxidative stress. Arch. Biochem. Biophys. 367, 216–224 (1999).
Toyokuni, S., Okamoto, K., Yodoi, J. & Hiai, H. Persistent oxidative stress in cancer. FEBS Lett. 358, 1–3 (1995).
Blackburn, R. V. et al. Metabolic oxidative stress activates signal transduction and gene expression during glucose deprivation in human tumor cells. Free Radic. Biol. Med. 26, 419–430 (1999).
Toffoli, G. et al. Expression of glutathione S-transferases in human tumors. Eur. J. Cancer 28, 1441–1446 (1992).
Di Illio, C. et al. Glutathione transferase isoenzymes in normal and neoplastic kidney tissue. Carcinogenesis 12, 1471–1475 (1991).
Giralt, M., Lafuente, A., Pujol, F. & Mallol, J. Enhanced glutathione S-transferase activity and glutathione content in human bladder cancer. Follow up study: influence of smoking. J. Urol. 149, 1452–1454 (1993).
Savic-Radojevic, A. et al. Glutathione S-transferase-P1 expression correlates with increased antioxidant capacity in transitional cell carcinoma of the urinary bladder. Eur. Urol. 52, 470–477 (2007).
Goldschmidt-Clermont, P. & Moldovan, L. Stress, superoxide and signal transduction. Gene Expr. 7, 255–260 (1999).
Yang, C. R. et al. Intracellular glutathione content of urothelial cancer in correlation to chemotherapy response. Cancer Lett. 119, 157–162 (1997).
O'Brien, M. L. & Tew, K. D. Glutathione and related enzymes in multidrug resistance. Eur. J. Cancer 32, 967–978 (1996).
Wang, T., Arifoglu, P., Ronai, Z. & Tew, K. Glutathione S-transferase P1–1 (GSTP1–1) inhibits c-Jun N-terminal kinase (JNK1) signaling through interaaction with the C terminus. J. Biol. Chem. 276, 20999–21003 (2001).
Wada, T. & Penninger, J. Mitogen-activated protein kinases in apoptosis regulation. Oncogene 23, 2838–2849 (2004).
Burg, D., Riepsaame, J., Pont, C., Mulder, G. & van de Water, B. Peptide-bond modified glutathione conjugate analogs modulate GST pi function in glutathione-conjugation, drug sensitivity and JNK signaling. Biochem. Pharmacol. 71, 268–277 (2006).
Pljesa-Ercegovac, M. et al. Enhanced GSTP1 expression in transitional cell carcinoma of urinary bladder is associated with altered apoptotic pathways. Urol. Oncol. doi:10.1016/j.urolonc.2008.10.019 (2009).
Lo, H. W. & Ali-Osman, F. Genetic polymorphism and function of glutathione S-transferases in tumor drug resistance. Curr. Opin. Pharmacol. 7, 367–374 (2007).
Yu, D. S., Ma, C. P. & Chang, S. Y. Establishment and characterization of renal cell carcinoma cell lines with multidrug resistance. Urol. Res. 28, 86–92 (2000).
Volm, M., Kastel. M. & Mattern Jefferth, T. Expression of resistance factors (P-glycoprotein, glutathione S-transferase-pi, and topoisomerase II) and their interrelationship to proto-oncogene products in renal cell carcinomas. Cancer 71, 3981–3987 (1993).
Hussain, S. A. & James, N. D. Molecular markers in bladder cancer. Semin. Radiat. Oncol. 15, 3–9 (2005).
Tew, K. D., Dutta, S. & Schultz, M. Inhibitors of glutathione S-transferases as therapeutic agents. Adv. Drug Deliv. Rev. 26, 91–104 (1997).
Adler, V. et al. Regulation of JNK signaling by GSTp. EMBO J. 18, 1321–1334 (1999).
Tew, K. D. TLK-286: a novel glutathione S-transferase-activated prodrug. Expert Opin. Investig. Drugs 14, 1047–1054 (2005).
Vergote, I. et al. Single agent, canfosfamide (C, TLK286) vs pegylated liposomal doxorubicin (D) or topotecan (T) in 3rd-line treatment of platinum (P) refractory or resistant ovarian cancer (OC): phase 3 study results [Abstract]. J. Clin. Oncol. 25 (18 Suppl.), LBA5528 (2007).
Byun, S. S., Kim, S. W., Choi, H., Lee, C. & Lee, E. Augmentation of cisplatin sensitivity in cisplatin-resistant human bladder cancer cells by modulating glutathione concentrations and glutathione-related enzyme activities. BJU Int. 95, 1086–1990 (2005).
Lafuente, A. et al. Limitations in the use of glutathione S-transferase P1 in urine as a marker for bladder cancer. Anticancer Res. 18, 3771–3772 (1998).
Berendsen, C. L., Mulder, T. P. & Peters, W. H. Plasma glutathione S-transferase pi 1–1 AND alpha 1–1 levels in patients with bladder cancer. J. Urol. 164, 2126–2128 (2000).
Stenzl, A. et al. The updated EAU guidelines on muscle-invasive and metastatic bladder cancer. Eur. Urol. 55, 815–825 (2009).
Acknowledgements
This work was supported by a grant (145009DJ) from the Serbian Ministry of Science and Technology.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Simic, T., Savic-Radojevic, A., Pljesa-Ercegovac, M. et al. Glutathione S-transferases in kidney and urinary bladder tumors. Nat Rev Urol 6, 281–289 (2009). https://doi.org/10.1038/nrurol.2009.49
Issue Date:
DOI: https://doi.org/10.1038/nrurol.2009.49
This article is cited by
-
GSTA4 mediates reduction of cisplatin ototoxicity in female mice
Nature Communications (2019)
-
MicroRNA profiling associated with muscle growth in modern broilers compared to an unselected chicken breed
BMC Genomics (2018)
-
A redox state-dictated signalling pathway deciphers the malignant cell specificity of CD40-mediated apoptosis
Oncogene (2017)
-
Copper-Induced Inactivation of Camel Liver Glutathione S-Transferase
Biological Trace Element Research (2016)
-
Cytoprotective and regulatory functions of glutathione S-transferases in cancer cell proliferation and cell death
Cancer Chemotherapy and Pharmacology (2015)