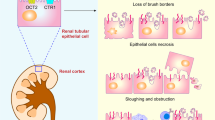
Abstract
Cisplatin is an effective chemotherapeutic agent for various solid tumours, but its use is limited by adverse effects in normal tissues. In particular, cisplatin is nephrotoxic and can cause acute kidney injury and chronic kidney disease. Preclinical studies have provided insights into the cellular and molecular mechanisms of cisplatin nephrotoxicity, which involve intracellular stresses including DNA damage, mitochondrial pathology, oxidative stress and endoplasmic reticulum stress. Stress responses, including autophagy, cell-cycle arrest, senescence, apoptosis, programmed necrosis and inflammation have key roles in the pathogenesis of cisplatin nephrotoxicity. In addition, emerging evidence suggests a contribution of epigenetic changes to cisplatin-induced acute kidney injury and chronic kidney disease. Further research is needed to determine how these pathways are integrated and to identify the cell type-specific roles of critical molecules involved in regulated necrosis, inflammation and epigenetic modifications in cisplatin nephrotoxicity. A number of potential therapeutic targets for cisplatin nephrotoxicity have been identified. However, the effects of renoprotective strategies on the efficacy of cisplatin chemotherapy needs to be thoroughly evaluated. Further research using tumour-bearing animals, multi-omics and genome-wide association studies will enable a comprehensive understanding of the complex cellular and molecular mechanisms of cisplatin nephrotoxicity and potentially lead to the identification of specific targets to protect the kidney without compromising the chemotherapeutic efficacy of cisplatin.
Key points
-
Cisplatin is nephrotoxin that can cause both acute kidney injury and chronic kidney disease.
-
Accumulation of cisplatin in renal tubular cells induces various intracellular stresses, including DNA damage, mitochondrial pathology, oxidative stress and endoplasmic reticulum stress.
-
Multiple stress responses, including autophagy, cell-cycle arrest, senescence, apoptosis, programmed necrosis and inflammation, have important roles in the pathogenesis of cisplatin nephrotoxicity.
-
Epigenetic mechanisms, including histone acetylation, DNA and messenger RNA methylation and gene regulation by non-coding RNAs also contribute to cisplatin nephrotoxicity.
-
How the various cellular stresses and pathways are integrated and the cell type-specific roles of critical molecules involved in regulated necrosis, inflammation and epigenetic modifications in cisplatin nephrotoxicity remain to be determined.
-
Renoprotective strategies for cisplatin nephrotoxicity have been identified in preclinical studies; however, their effects on the efficacy of cisplatin-mediated cancer therapy in animal models must be evaluated before clinical translation.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Dasari, S. & Tchounwou, P. B. Cisplatin in cancer therapy: molecular mechanisms of action. Eur. J. Pharmacol. 740, 364–378 (2014).
Klumpers, M. J. et al. Genome-wide analyses of nephrotoxicity in platinum-treated cancer patients identify association with genetic variant in RBMS3 and acute kidney injury. J. Pers. Med. 12, 892 (2022).
Volarevic, V. et al. Molecular mechanisms of cisplatin-induced nephrotoxicity: a balance on the knife edge between renoprotection and tumor toxicity. J. Biomed. Sci. 26, 25 (2019).
Miller, R. P., Tadagavadi, R. K., Ramesh, G. & Reeves, W. B. Mechanisms of cisplatin nephrotoxicity. Toxins 2, 2490–2518 (2010).
Latcha, S. et al. Long-term renal outcomes after cisplatin treatment. Clin. J. Am. Soc. Nephrol. 11, 1173–1179 (2016).
Holditch, S. J., Brown, C. N., Lombardi, A. M., Nguyen, K. N. & Edelstein, C. L. Recent advances in models, mechanisms, biomarkers, and interventions in cisplatin-induced acute kidney injury. Int. J. Mol. Sci. 20, 3011 (2019).
Katagiri, D. et al. Interstitial renal fibrosis due to multiple cisplatin treatments is ameliorated by semicarbazide-sensitive amine oxidase inhibition. Kidney Int. 89, 374–385 (2016).
Sharp, C. N. et al. Repeated administration of low-dose cisplatin in mice induces fibrosis. Am. J. Physiol. Renal Physiol. 310, F560–F568 (2016).
Torres, R. et al. Three-dimensional morphology by multiphoton microscopy with clearing in a model of cisplatin-induced CKD. J. Am. Soc. Nephrol. 27, 1102–1112 (2016).
Sharp, C. N. et al. Subclinical kidney injury induced by repeated cisplatin administration results in progressive chronic kidney disease. Am. J. Physiol. Renal Physiol. 315, F161–F172 (2018).
Fu, Y. et al. Chronic effects of repeated low-dose cisplatin treatment in mouse kidneys and renal tubular cells. Am. J. Physiol. Renal Physiol. 317, F1582–F1592 (2019).
Landau, S. I. et al. Regulated necrosis and failed repair in cisplatin-induced chronic kidney disease. Kidney Int. 95, 797–814 (2019).
Menshikh, A. et al. Capillary rarefaction is more closely associated with CKD progression after cisplatin, rhabdomyolysis, and ischemia-reperfusion-induced AKI than renal fibrosis. Am. J. Physiol. Renal Physiol. 317, F1383–F1397 (2019).
Sears, S. M. et al. C57BL/6 mice require a higher dose of cisplatin to induce renal fibrosis and CCL2 correlates with cisplatin-induced kidney injury. Am. J. Physiol. Renal Physiol. 319, F674–F685 (2020).
Black, L. M. et al. Divergent effects of AKI to CKD models on inflammation and fibrosis. Am. J. Physiol. Renal Physiol. 315, F1107–F1118 (2018).
Sharp, C. N., Doll, M., Dupre, T. V., Beverly, L. J. & Siskind, L. J. Moderate aging does not exacerbate cisplatin-induced kidney injury or fibrosis despite altered inflammatory cytokine expression and immune cell infiltration. Am. J. Physiol. Renal Physiol. 316, F162–F172 (2019).
Fujishiro, H., Taguchi, H., Hamao, S., Sumi, D. & Himeno, S. Comparisons of segment-specific toxicity of platinum-based agents and cadmium using S1, S2, and S3 cells derived from mouse kidney proximal tubules. Toxicol. Vitr. 75, 105179 (2021).
Filipski, K. K., Mathijssen, R. H., Mikkelsen, T. S., Schinkel, A. H. & Sparreboom, A. Contribution of organic cation transporter 2 (OCT2) to cisplatin-induced nephrotoxicity. Clin. Pharmacol. Ther. 86, 396–402 (2009).
Ciarimboli, G. et al. Organic cation transporter 2 mediates cisplatin-induced oto- and nephrotoxicity and is a target for protective interventions. Am. J. Pathol. 176, 1169–1180 (2010).
Guo, D. et al. Selective inhibition on organic cation transporters by carvedilol protects mice from cisplatin-induced nephrotoxicity. Pharm. Res. 35, 204 (2018).
Hu, S. et al. Identification of OAT1/OAT3 as contributors to cisplatin toxicity. Clin. Transl. Sci. 10, 412–420 (2017).
Pabla, N., Murphy, R. F., Liu, K. & Dong, Z. The copper transporter Ctr1 contributes to cisplatin uptake by renal tubular cells during cisplatin nephrotoxicity. Am. J. Physiol. Renal Physiol. 296, F505–F511 (2009).
Wen, X. et al. Transgenic expression of the human MRP2 transporter reduces cisplatin accumulation and nephrotoxicity in Mrp2-null mice. Am. J. Pathol. 184, 1299–1308 (2014).
Nakamura, T., Yonezawa, A., Hashimoto, S., Katsura, T. & Inui, K. Disruption of multidrug and toxin extrusion MATE1 potentiates cisplatin-induced nephrotoxicity. Biochem. Pharmacol. 80, 1762–1767 (2010).
Mizuno, T. et al. Significance of downregulation of renal organic cation transporter (SLC47A1) in cisplatin-induced proximal tubular injury. Onco Targets Ther. 8, 1701–1706 (2015).
Ciarimboli, G. Membrane transporters as mediators of cisplatin side-effects. Anticancer. Res. 34, 547–550 (2014).
Katsuda, H. et al. Protecting cisplatin-induced nephrotoxicity with cimetidine does not affect antitumor activity. Biol. Pharm. Bull. 33, 1867–1871 (2010).
Iwata, K. et al. Effects of genetic variants in SLC22A2 organic cation transporter 2 and SLC47A1 multidrug and toxin extrusion 1 transporter on cisplatin-induced adverse events. Clin. Exp. Nephrol. 16, 843–851 (2012).
Zhang, J. & Zhou, W. Ameliorative effects of SLC22A2 gene polymorphism 808 G/T and cimetidine on cisplatin-induced nephrotoxicity in Chinese cancer patients. Food Chem. Toxicol. 50, 2289–2293 (2012).
Ghonaim, E., El-Haggar, S. & Gohar, S. Possible protective effect of pantoprazole against cisplatin-induced nephrotoxicity in head and neck cancer patients: a randomized controlled trial. Med. Oncol. 38, 108 (2021).
Fox, E. et al. Pantoprazole, an inhibitor of the organic cation transporter 2, does not ameliorate cisplatin-related ototoxicity or nephrotoxicity in children and adolescents with newly diagnosed osteosarcoma treated with methotrexate, doxorubicin, and cisplatin. Oncologist 23, 762–e779 (2018).
Solanki, M. H. et al. Magnesium protects against cisplatin-induced acute kidney injury without compromising cisplatin-mediated killing of an ovarian tumor xenograft in mice. Am. J. Physiol. Renal Physiol. 309, F35–F47 (2015).
Kumar, G. et al. Magnesium improves cisplatin-mediated tumor killing while protecting against cisplatin-induced nephrotoxicity. Am. J. Physiol. Renal Physiol. 313, F339–F350 (2017).
Saito, Y. et al. Magnesium attenuates cisplatin-induced nephrotoxicity by regulating the expression of renal transporters. Eur. J. Pharmacol. 811, 191–198 (2017).
Kimura, T. et al. Renal protective effect of a hydration supplemented with magnesium in patients receiving cisplatin for head and neck cancer. J. Otolaryngol. Head. Neck Surg. 47, 10 (2018).
Yamamoto, Y. et al. Nephroprotective effects of hydration with magnesium in patients with cervical cancer receiving cisplatin. Anticancer. Res. 35, 2199–2204 (2015).
Saito, Y. et al. Premedication with intravenous magnesium has a protective effect against cisplatin-induced nephrotoxicity. Support. Care Cancer 25, 481–487 (2017).
Saito, Y. et al. Magnesium co-administration decreases cisplatin-induced nephrotoxicity in the multiple cisplatin administration. Life Sci. 189, 18–22 (2017).
Townsend, D. M., Deng, M., Zhang, L., Lapus, M. G. & Hanigan, M. H. Metabolism of cisplatin to a nephrotoxin in proximal tubule cells. J. Am. Soc. Nephrol. 14, 1–10 (2003).
Hanigan, M. H. et al. Gamma-glutamyl transpeptidase-deficient mice are resistant to the nephrotoxic effects of cisplatin. Am. J. Pathol. 159, 1889–1894 (2001).
Hanigan, M. H., Gallagher, B. C., Taylor, P. T. Jr & Large, M. K. Inhibition of gamma-glutamyl transpeptidase activity by acivicin in vivo protects the kidney from cisplatin-induced toxicity. Cancer Res. 54, 5925–5929 (1994).
Townsend, D. M. & Hanigan, M. H. Inhibition of gamma-glutamyl transpeptidase or cysteine S-conjugate beta-lyase activity blocks the nephrotoxicity of cisplatin in mice. J. Pharmacol. Exp. Ther. 300, 142–148 (2002).
Basu, A. & Krishnamurthy, S. Cellular responses to cisplatin-induced DNA damage. J. Nucleic Acids 2010, 201367 (2010).
Wang, D. & Lippard, S. J. Cellular processing of platinum anticancer drugs. Nat. Rev. Drug Discov. 4, 307–320 (2005).
Jackson, S. P. & Bartek, J. The DNA-damage response in human biology and disease. Nature 461, 1071–1078 (2009).
Khrunin, A. et al. Pharmacogenomics of cisplatin-based chemotherapy in ovarian cancer patients of different ethnic origins. Pharmacogenomics 13, 171–178 (2012).
Tzvetkov, M. V. et al. Pharmacogenetic analyses of cisplatin-induced nephrotoxicity indicate a renoprotective effect of ERCC1 polymorphisms. Pharmacogenomics 12, 1417–1427 (2011).
Zazuli, Z. et al. Outcome definition influences the relationship between genetic polymorphisms of ERCC1, ERCC2, SLC22A2 and cisplatin nephrotoxicity in adult testicular cancer patients. Genes 10, 364 (2019).
Zazuli, Z. et al. Genetic variations and cisplatin nephrotoxicity: a systematic review. Front. Pharmacol. 9, 1111 (2018).
Morizane, R. et al. Nephron organoids derived from human pluripotent stem cells model kidney development and injury. Nat. Biotechnol. 33, 1193–1200 (2015).
Pabla, N., Huang, S., Mi, Q. S., Daniel, R. & Dong, Z. ATR-Chk2 signaling in p53 activation and DNA damage response during cisplatin-induced apoptosis. J. Biol. Chem. 283, 6572–6583 (2008).
Kishi, S. et al. Proximal tubule ATR regulates DNA repair to prevent maladaptive renal injury responses. J. Clin. Invest. 129, 4797–4816 (2019).
Wang, J. et al. Caspase-mediated cleavage of ATM during cisplatin-induced tubular cell apoptosis: inactivation of its kinase activity toward p53. Am. J. Physiol. Renal Physiol. 291, F1300–F1307 (2006).
Uehara, M. et al. Pharmacological inhibition of ataxia-telangiectasia mutated exacerbates acute kidney injury by activating p53 signaling in mice. Sci. Rep. 10, 4441 (2020).
Yan, M., Tang, C., Ma, Z., Huang, S. & Dong, Z. DNA damage response in nephrotoxic and ischemic kidney injury. Toxicol. Appl. Pharmacol. 313, 104–108 (2016).
Yamashita, N. et al. Cumulative DNA damage by repeated low-dose cisplatin injection promotes the transition of acute to chronic kidney injury in mice. Sci. Rep. 11, 20920 (2021).
Gupta, N. et al. Modeling injury and repair in kidney organoids reveals that homologous recombination governs tubular intrinsic repair. Sci. Transl. Med. 14, eabj4772 (2022).
Rundle, S., Bradbury, A., Drew, Y. & Curtin, N. J. Targeting the ATR-CHK1 axis in cancer therapy. Cancers 9, 41 (2017).
Tang, C. et al. Mitochondrial quality control in kidney injury and repair. Nat. Rev. Nephrol. 17, 299–318 (2021).
Brooks, C., Wei, Q., Cho, S. G. & Dong, Z. Regulation of mitochondrial dynamics in acute kidney injury in cell culture and rodent models. J. Clin. Invest. 119, 1275–1285 (2009).
Morigi, M. et al. Sirtuin 3-dependent mitochondrial dynamic improvements protect against acute kidney injury. J. Clin. Invest. 125, 715–726 (2015).
Liu, Z. et al. Numb depletion promotes Drp1-mediated mitochondrial fission and exacerbates mitochondrial fragmentation and dysfunction in acute kidney injury. Antioxid. Redox Signal. 30, 1797–1816 (2019).
Chang, C. R. & Blackstone, C. Dynamic regulation of mitochondrial fission through modification of the dynamin-related protein Drp1. Ann. N. Y. Acad. Sci. 1201, 34–39 (2010).
Yuan, L. et al. PGC-1alpha alleviates mitochondrial dysfunction via TFEB-mediated autophagy in cisplatin-induced acute kidney injury. Aging 13, 8421–8439 (2021).
Lu, Q. et al. Rheb1 protects against cisplatin-induced tubular cell death and acute kidney injury via maintaining mitochondrial homeostasis. Cell Death Dis. 11, 364 (2020).
Oh, C. J. et al. Pyruvate dehydrogenase kinase 4 deficiency attenuates cisplatin-induced acute kidney injury. Kidney Int. 91, 880–895 (2017).
Yang, S. K. et al. Mitochondria targeted peptide SS-31 prevent on cisplatin-induced acute kidney injury via regulating mitochondrial ROS-NLRP3 pathway. Biomed. Pharmacother. 130, 110521 (2020).
Hu, Y. et al. Cisplatin-mediated upregulation of APE2 binding to MYH9 provokes mitochondrial fragmentation and acute kidney injury. Cancer Res. 81, 713–723 (2021).
Chung, K. W. et al. Mitochondrial damage and activation of the STING pathway lead to renal inflammation and fibrosis. Cell Metab. 30, 784–799 e785 (2019).
Wang, Y. et al. PINK1/Parkin-mediated mitophagy is activated in cisplatin nephrotoxicity to protect against kidney injury. Cell Death Dis. 9, 1113 (2018).
Fujii, R. et al. Decreased IFT88 expression with primary cilia shortening causes mitochondrial dysfunction in cisplatin-induced tubular injury. Am. J. Physiol. Renal Physiol. 321, F278–F292 (2021).
Wang, S., Zhuang, S. & Dong, Z. IFT88 deficiency in proximal tubular cells exaggerates cisplatin-induced injury by suppressing autophagy. Am. J. Physiol. Renal Physiol. 321, F269–F277 (2021).
Mapuskar, K. A. et al. Persistent increase in mitochondrial superoxide mediates cisplatin-induced chronic kidney disease. Redox Biol. 20, 98–106 (2019).
Quintanilha, J. C. F. et al. Involvement of cytochrome P450 in cisplatin treatment: implications for toxicity. Cancer Chemother. Pharmacol. 80, 223–233 (2017).
Pabla, N. & Dong, Z. Cisplatin nephrotoxicity: mechanisms and renoprotective strategies. Kidney Int. 73, 994–1007 (2008).
Hasegawa, K. et al. Kidney-specific overexpression of Sirt1 protects against acute kidney injury by retaining peroxisome function. J. Biol. Chem. 285, 13045–13056 (2010).
Chiba, T. et al. Sirtuin 5 regulates proximal tubule fatty acid oxidation to protect against AKI. J. Am. Soc. Nephrol. 30, 2384–2398 (2019).
Dutta, R. K. et al. Beneficial effects of myo-inositol oxygenase deficiency in cisplatin-induced AKI. J. Am. Soc. Nephrol. 28, 1421–1436 (2017).
Xiao, W., Wang, R. S., Handy, D. E. & Loscalzo, J. NAD(H) and NADP(H) redox couples and cellular energy metabolism. Antioxid. Redox Signal. 28, 251–272 (2018).
Oh, G. S. et al. Pharmacological activation of NQO1 increases NAD+ levels and attenuates cisplatin-mediated acute kidney injury in mice. Kidney Int. 85, 547–560 (2014).
Tran, M. T. et al. PGC1α drives NAD biosynthesis linking oxidative metabolism to renal protection. Nature 531, 528–532 (2016).
Katsyuba, E. et al. De novo NAD+ synthesis enhances mitochondrial function and improves health. Nature 563, 354–359 (2018).
Meng, X. M. et al. NADPH oxidase 4 promotes cisplatin-induced acute kidney injury via ROS-mediated programmed cell death and inflammation. Lab. Invest. 98, 63–78 (2018).
Casanova, A. G. et al. A meta-analysis of preclinical studies using antioxidants for the prevention of cisplatin nephrotoxicity: implications for clinical application. Crit. Rev. Toxicol. 50, 780–800 (2020).
Mukhopadhyay, P. et al. Mitochondrial-targeted antioxidants represent a promising approach for prevention of cisplatin-induced nephropathy. Free. Radic. Biol. Med. 52, 497–506 (2012).
Suzuki, T. et al. Mitochonic acid 5 binds mitochondria and ameliorates renal tubular and cardiac myocyte damage. J. Am. Soc. Nephrol. 27, 1925–1932 (2016).
Sanchez-Gonzalez, P. D. et al. Differential effect of quercetin on cisplatin-induced toxicity in kidney and tumor tissues. Food Chem. Toxicol. 107, 226–236 (2017).
Sanchez-Gonzalez, P. D., Lopez-Hernandez, F. J., Perez-Barriocanal, F., Morales, A. I. & Lopez-Novoa, J. M. Quercetin reduces cisplatin nephrotoxicity in rats without compromising its anti-tumour activity. Nephrol. Dial. Transpl. 26, 3484–3495 (2011).
Orsolic, N. & Car, N. Quercetin and hyperthermia modulate cisplatin-induced DNA damage in tumor and normal tissues in vivo. Tumour Biol. 35, 6445–6454 (2014).
Li, C., Shen, Y., Huang, L., Liu, C. & Wang, J. Senolytic therapy ameliorates renal fibrosis postacute kidney injury by alleviating renal senescence. FASEB J. 35, e21229 (2021).
Hetz, C., Zhang, K. & Kaufman, R. J. Mechanisms, regulation and functions of the unfolded protein response. Nat. Rev. Mol. Cell Biol. 21, 421–438 (2020).
Huang, Z. et al. Activation of GPR120 by TUG891 ameliorated cisplatin-induced acute kidney injury via repressing ER stress and apoptosis. Biomed. Pharmacother. 126, 110056 (2020).
Peyrou, M., Hanna, P. E. & Cribb, A. E. Cisplatin, gentamicin, and p-aminophenol induce markers of endoplasmic reticulum stress in the rat kidneys. Toxicol. Sci. 99, 346–353 (2007).
Yan, M., Shu, S., Guo, C., Tang, C. & Dong, Z. Endoplasmic reticulum stress in ischemic and nephrotoxic acute kidney injury. Ann. Med. 50, 381–390 (2018).
Kim, H. J., Yoon, Y. M., Lee, J. H. & Lee, S. H. Protective role of fucoidan on cisplatin-mediated ER stress in renal proximal tubule epithelial cells. Anticancer. Res. 39, 5515–5524 (2019).
Chen, B. et al. Epigallocatechin-3-gallate protects against cisplatin-induced nephrotoxicity by inhibiting endoplasmic reticulum stress-induced apoptosis. Exp. Biol. Med. 240, 1513–1519 (2015).
Hao, Y. et al. 2-Methylquinazoline derivative 23BB as a highly selective histone deacetylase 6 inhibitor alleviated cisplatin-induced acute kidney injury. Biosci. Rep. 40, BSR20191538 (2020).
Kong, D. et al. Erythropoietin protects against cisplatin-induced nephrotoxicity by attenuating endoplasmic reticulum stress-induced apoptosis. J. Nephrol. 26, 219–227 (2013).
Peyrou, M. & Cribb, A. E. Effect of endoplasmic reticulum stress preconditioning on cytotoxicity of clinically relevant nephrotoxins in renal cell lines. Toxicol. Vitr. 21, 878–886 (2007).
Shu, S. et al. Endoplasmic reticulum stress contributes to cisplatin-induced chronic kidney disease via the PERK-PKCδ pathway. Cell Mol. Life Sci. 79, 452 (2022).
Periyasamy-Thandavan, S. et al. Autophagy is cytoprotective during cisplatin injury of renal proximal tubular cells. Kidney Int. 74, 631–640 (2008).
Yang, C., Kaushal, V., Shah, S. V. & Kaushal, G. P. Autophagy is associated with apoptosis in cisplatin injury to renal tubular epithelial cells. Am. J. Physiol. Renal Physiol. 294, F777–F787 (2008).
Inoue, K. et al. Cisplatin-induced macroautophagy occurs prior to apoptosis in proximal tubules in vivo. Clin. Exp. Nephrol. 14, 112–122 (2010).
Jiang, M. et al. Autophagy in proximal tubules protects against acute kidney injury. Kidney Int. 82, 1271–1283 (2012).
Takahashi, A. et al. Autophagy guards against cisplatin-induced acute kidney injury. Am. J. Pathol. 180, 517–525 (2012).
Hu, X., Ma, Z., Wen, L., Li, S. & Dong, Z. Autophagy in cisplatin nephrotoxicity during cancer therapy. Cancers 13, 5618 (2021).
Liu, J. et al. Histone deacetylase inhibitors protect against cisplatin-induced acute kidney injury by activating autophagy in proximal tubular cells. Cell Death Dis. 9, 322 (2018).
Zhou, Q. et al. Safety profile of rapamycin perfluorocarbon nanoparticles for preventing cisplatin-induced kidney injury. Nanomaterials 12, 336 (2022).
Wei, L. et al. AMP-activated protein kinase regulates autophagic protection against cisplatin-induced tissue injury in the kidney. Genet. Mol. Res. 14, 12006–12015 (2015).
Li, J. et al. Rictor/mTORC2 protects against cisplatin-induced tubular cell death and acute kidney injury. Kidney Int. 86, 86–102 (2014).
Kimura, A. et al. Interferon-gamma is protective in cisplatin-induced renal injury by enhancing autophagic flux. Kidney Int. 82, 1093–1104 (2012).
Shen, Q. et al. TLR2 protects cisplatin-induced acute kidney injury associated with autophagy via PI3K/Akt signaling pathway. J. Cell Biochem. 120, 4366–4374 (2019).
Zhao, C. et al. Drp1-dependent mitophagy protects against cisplatin-induced apoptosis of renal tubular epithelial cells by improving mitochondrial function. Oncotarget 8, 20988–21000 (2017).
Lynch, M. R. et al. TFEB-driven lysosomal biogenesis is pivotal for PGC1alpha-dependent renal stress resistance. JCI Insight 5, e126749 (2020).
Fu, Y. et al. Persistent activation of autophagy after cisplatin nephrotoxicity promotes renal fibrosis and chronic kidney disease. Front. Pharmacol. 13, 918732 (2022).
Sears, S. M. et al. Pharmacologic inhibitors of autophagy have opposite effects in acute and chronic cisplatin-induced kidney injury. Am. J. Physiol. Renal Physiol. https://doi.org/10.1152/ajprenal.00097.2022 (2022).
Shi, M. et al. In vivo evidence for therapeutic applications of beclin 1 to promote recovery and inhibit fibrosis after acute kidney injury. Kidney Int. 101, 63–78 (2022).
Minami, S. & Nakamura, S. Therapeutic potential of beclin1 for transition from AKI to CKD: autophagy-dependent and autophagy-independent functions. Kidney Int. 101, 13–15 (2022).
Baisantry, A. et al. Autophagy induces prosenescent changes in proximal tubular S3 segments. J. Am. Soc. Nephrol. 27, 1609–1616 (2016).
Livingston, M. J. et al. Tubular cells produce FGF2 via autophagy after acute kidney injury leading to fibroblast activation and renal fibrosis. Autophagy https://doi.org/10.1080/15548627.2022.2072054 (2022).
Li, L., Wang, Z. V., Hill, J. A. & Lin, F. New autophagy reporter mice reveal dynamics of proximal tubular autophagy. J. Am. Soc. Nephrol. 25, 305–315 (2014).
Hu, X. et al. FGF2 is produced by renal tubular cells to act as a paracrine factor in maladaptive kidney repair after cisplatin nephrotoxicity. Lab Invest. (2022).
Panagopoulos, A. & Altmeyer, M. The hammer and the dance of cell cycle control. Trends Biochem. Sci. 46, 301–314 (2021).
Megyesi, J., Safirstein, R. L. & Price, P. M. Induction of p21WAF1/CIP1/SDI1 in kidney tubule cells affects the course of cisplatin-induced acute renal failure. J. Clin. Invest. 101, 777–782 (1998).
Price, P. M., Safirstein, R. L. & Megyesi, J. Protection of renal cells from cisplatin toxicity by cell cycle inhibitors. Am. J. Physiol. Renal Physiol. 286, F378–F384 (2004).
Yu, F., Megyesi, J., Safirstein, R. L. & Price, P. M. Identification of the functional domain of p21(WAF1/CIP1) that protects cells from cisplatin cytotoxicity. Am. J. Physiol. Renal Physiol. 289, F514–F520 (2005).
Price, P. M. et al. Dependence of cisplatin-induced cell death in vitro and in vivo on cyclin-dependent kinase 2. J. Am. Soc. Nephrol. 17, 2434–2442 (2006).
Yu, F., Megyesi, J., Safirstein, R. L. & Price, P. M. Involvement of the CDK2-E2F1 pathway in cisplatin cytotoxicity in vitro and in vivo. Am. J. Physiol. Renal Physiol. 293, F52–F59 (2007).
Hodeify, R., Tarcsafalvi, A., Megyesi, J., Safirstein, R. L. & Price, P. M. Cdk2-dependent phosphorylation of p21 regulates the role of Cdk2 in cisplatin cytotoxicity. Am. J. Physiol. Renal Physiol. 300, F1171–F1179 (2011).
Miyaji, T., Kato, A., Yasuda, H., Fujigaki, Y. & Hishida, A. Role of the increase in p21 in cisplatin-induced acute renal failure in rats. J. Am. Soc. Nephrol. 12, 900–908 (2001).
Megyesi, J., Andrade, L., Vieira, J. M. Jr, Safirstein, R. L. & Price, P. M. Coordination of the cell cycle is an important determinant of the syndrome of acute renal failure. Am. J. Physiol. Renal Physiol. 283, F810–F816 (2002).
DiRocco, D. P. et al. CDK4/6 inhibition induces epithelial cell cycle arrest and ameliorates acute kidney injury. Am. J. Physiol. Renal Physiol. 306, F379–F388 (2014).
Pabla, N. et al. Mitigation of acute kidney injury by cell-cycle inhibitors that suppress both CDK4/6 and OCT2 functions. Proc. Natl Acad. Sci. USA 112, 5231–5236 (2015).
Kim, J. Y. et al. Ribociclib mitigates cisplatin-associated kidney injury through retinoblastoma-1 dependent mechanisms. Biochem. Pharmacol. 177, 113939 (2020).
Jin, H. et al. Epithelial innate immunity mediates tubular cell senescence after kidney injury. JCI Insight 4, e125490 (2019).
Li, C. et al. N-acetylcysteine ameliorates cisplatin-induced renal senescence and renal interstitial fibrosis through sirtuin1 activation and p53 deacetylation. Free Radic. Biol. Med. 130, 512–527 (2019).
Hickson, L. J. et al. Senolytics decrease senescent cells in humans: preliminary report from a clinical trial of dasatinib plus quercetin in individuals with diabetic kidney disease. EBioMedicine 47, 446–456 (2019).
Duan, Z., Cai, G., Li, J. & Chen, X. Cisplatin-induced renal toxicity in elderly people. Ther. Adv. Med. Oncol. 12, 1758835920923430 (2020).
Guan, Y. et al. Nicotinamide mononucleotide, an NAD+ precursor, rescues age-associated susceptibility to AKI in a sirtuin 1-dependent manner. J. Am. Soc. Nephrol. 28, 2337–2352 (2017).
Wei, M. C. et al. Proapoptotic BAX and BAK: a requisite gateway to mitochondrial dysfunction and death. Science 292, 727–730 (2001).
Jiang, M., Wang, C. Y., Huang, S., Yang, T. & Dong, Z. Cisplatin-induced apoptosis in p53-deficient renal cells via the intrinsic mitochondrial pathway. Am. J. Physiol. Renal Physiol. 296, F983–F993 (2009).
Wei, Q., Dong, G., Franklin, J. & Dong, Z. The pathological role of Bax in cisplatin nephrotoxicity. Kidney Int. 72, 53–62 (2007).
Mihara, M. et al. p53 has a direct apoptogenic role at the mitochondria. Mol. Cell 11, 577–590 (2003).
Nakano, K. & Vousden, K. H. PUMA, a novel proapoptotic gene, is induced by p53. Mol. Cell. 7, 683–694 (2001).
Wei, Q. et al. Activation and involvement of p53 in cisplatin-induced nephrotoxicity. Am. J. Physiol. Renal Physiol. 293, F1282–F1291 (2007).
Zhang, D. et al. Tubular p53 regulates multiple genes to mediate AKI. J. Am. Soc. Nephrol. 25, 2278–2289 (2014).
Tsuruya, K. et al. Direct involvement of the receptor-mediated apoptotic pathways in cisplatin-induced renal tubular cell death. Kidney Int. 63, 72–82 (2003).
Linkermann, A. et al. Renal tubular Fas ligand mediates fratricide in cisplatin-induced acute kidney failure. Kidney Int. 79, 169–178 (2011).
Ramesh, G. & Reeves, W. B. TNFR2-mediated apoptosis and necrosis in cisplatin-induced acute renal failure. Am. J. Physiol. Renal Physiol. 285, F610–F618 (2003).
Iurlaro, R. & Munoz-Pinedo, C. Cell death induced by endoplasmic reticulum stress. FEBS J. 283, 2640–2652 (2016).
Li, F. et al. Compound C protects against cisplatin-induced nephrotoxicity through pleiotropic effects. Front. Physiol. 11, 614244 (2020).
Choi, M. E., Price, D. R., Ryter, S. W. & Choi, A. M. K. Necroptosis: a crucial pathogenic mediator of human disease. JCI Insight 4, e128834 (2019).
Xu, Y. et al. A role for tubular necroptosis in cisplatin-induced AKI. J. Am. Soc. Nephrol. 26, 2647–2658 (2015).
Ning, Y. et al. Necrostatin-1 attenuates cisplatin-induced nephrotoxicity through suppression of apoptosis and oxidative stress and retains Klotho expression. Front. Pharmacol. 9, 384 (2018).
Wang, J. N. et al. RIPK1 inhibitor Cpd-71 attenuates renal dysfunction in cisplatin-treated mice via attenuating necroptosis, inflammation and oxidative stress. Clin. Sci. 133, 1609–1627 (2019).
Guo, X. et al. Kidney-targeted renalase agonist prevents cisplatin-induced chronic kidney disease by inhibiting regulated necrosis and inflammation. J. Am. Soc. Nephrol. 33, 342–356 (2022).
Li, Y. et al. Activation of GSDMD contributes to acute kidney injury induced by cisplatin. Am. J. Physiol. Renal Physiol. 318, F96–F106 (2020).
Miao, N. et al. The cleavage of gasdermin D by caspase-11 promotes tubular epithelial cell pyroptosis and urinary IL-18 excretion in acute kidney injury. Kidney Int. 96, 1105–1120 (2019).
Xia, W. et al. Gasdermin E deficiency attenuates acute kidney injury by inhibiting pyroptosis and inflammation. Cell Death Dis. 12, 139 (2021).
Shen, X., Wang, H., Weng, C., Jiang, H. & Chen, J. Caspase 3/GSDME-dependent pyroptosis contributes to chemotherapy drug-induced nephrotoxicity. Cell Death Dis. 12, 186 (2021).
Jiang, X., Stockwell, B. R. & Conrad, M. Ferroptosis: mechanisms, biology and role in disease. Nat. Rev. Mol. Cell Biol. 22, 266–282 (2021).
Friedmann Angeli, J. P. et al. Inactivation of the ferroptosis regulator Gpx4 triggers acute renal failure in mice. Nat. Cell Biol. 16, 1180–1191 (2014).
Deng, F., Sharma, I., Dai, Y., Yang, M. & Kanwar, Y. S. Myo-inositol oxygenase expression profile modulates pathogenic ferroptosis in the renal proximal tubule. J. Clin. Invest. 129, 5033–5049 (2019).
Mishima, E. et al. Drugs repurposed as antiferroptosis agents suppress organ damage, including AKI, by functioning as lipid peroxyl radical scavengers. J. Am. Soc. Nephrol. 31, 280–296 (2020).
Ikeda, Y. et al. Role of ferroptosis in cisplatin-induced acute nephrotoxicity in mice. J. Trace Elem. Med. Biol. 67, 126798 (2021).
Hu, Z. et al. VDR activation attenuate cisplatin induced AKI by inhibiting ferroptosis. Cell Death Dis. 11, 73 (2020).
Zazuli, Z. et al. Association between genetic variants and cisplatin-induced nephrotoxicity: a genome-wide approach and validation study. J. Pers. Med. 11, 1233 (2021).
Andrade-Silva, M. et al. TLR2 and TLR4 play opposite role in autophagy associated with cisplatin-induced acute kidney injury. Clin. Sci. 132, 1725–1739 (2018).
Zhang, B., Ramesh, G., Uematsu, S., Akira, S. & Reeves, W. B. TLR4 signaling mediates inflammation and tissue injury in nephrotoxicity. J. Am. Soc. Nephrol. 19, 923–932 (2008).
Alikhan, M. A. et al. Endogenous toll-like receptor 9 regulates AKI by promoting regulatory T cell recruitment. J. Am. Soc. Nephrol. 27, 706–714 (2016).
Maekawa, H. et al. Mitochondrial damage causes inflammation via cGAS-STING signaling in acute kidney injury. Cell Rep. 29, 1261–1273 e1266 (2019).
Gong, W. et al. The novel STING antagonist H151 ameliorates cisplatin-induced acute kidney injury and mitochondrial dysfunction. Am. J. Physiol. Renal Physiol. 320, F608–F616 (2021).
Kim, H. J. et al. NLRP3 inflammasome knockout mice are protected against ischemic but not cisplatin-induced acute kidney injury. J. Pharmacol. Exp. Ther. 346, 465–472 (2013).
Li, S. et al. NLRP3 inflammasome inhibition attenuates cisplatin-induced renal fibrosis by decreasing oxidative stress and inflammation. Exp. Cell Res. 383, 111488 (2019).
Lv, L. L. et al. The pattern recognition receptor, Mincle, is essential for maintaining the M1 macrophage phenotype in acute renal inflammation. Kidney Int. 91, 587–602 (2017).
Holbrook, J., Lara-Reyna, S., Jarosz-Griffiths, H. & McDermott, M. Tumour necrosis factor signalling in health and disease. F1000Res. https://doi.org/10.12688/f1000research.17023.1 (2019).
Dong, Z. & Atherton, S. S. Tumor necrosis factor-alpha in cisplatin nephrotoxicity: a homebred foe? Kidney Int. 72, 5–7 (2007).
Ramesh, G. & Reeves, W. B. TNF-alpha mediates chemokine and cytokine expression and renal injury in cisplatin nephrotoxicity. J. Clin. Invest. 110, 835–842 (2002).
Zhang, J. et al. Competing actions of type 1 angiotensin II receptors expressed on T lymphocytes and kidney epithelium during cisplatin-induced AKI. J. Am. Soc. Nephrol. 27, 2257–2264 (2016).
Liu, M. et al. A pathophysiologic role for T lymphocytes in murine acute cisplatin nephrotoxicity. J. Am. Soc. Nephrol. 17, 765–774 (2006).
Lee, H. et al. CD4+CD25+ regulatory T cells attenuate cisplatin-induced nephrotoxicity in mice. Kidney Int. 78, 1100–1109 (2010).
Faubel, S. et al. Cisplatin-induced acute renal failure is associated with an increase in the cytokines interleukin (IL)-1β, IL-18, IL-6, and neutrophil infiltration in the kidney. J. Pharmacol. Exp. Ther. 322, 8–15 (2007).
Privratsky, J. R. et al. Interleukin 1 receptor (IL-1R1) activation exacerbates toxin-induced acute kidney injury. Am. J. Physiol. Renal Physiol. 315, F682–F691 (2018).
Nozaki, Y. et al. Signaling through the interleukin-18 receptor α attenuates inflammation in cisplatin-induced acute kidney injury. Kidney Int. 82, 892–902 (2012).
Mitazaki, S. et al. Interleukin-6 plays a protective role in development of cisplatin-induced acute renal failure through upregulation of anti-oxidative stress factors. Life Sci. 88, 1142–1148 (2011).
Mitazaki, S., Kato, N., Suto, M., Hiraiwa, K. & Abe, S. Interleukin-6 deficiency accelerates cisplatin-induced acute renal failure but not systemic injury. Toxicology 265, 115–121 (2009).
Tadagavadi, R. K. & Reeves, W. B. Endogenous IL-10 attenuates cisplatin nephrotoxicity: role of dendritic cells. J. Immunol. 185, 4904–4911 (2010).
Wang, W. W. et al. IL-10 from dendritic cells but not from T regulatory cells protects against cisplatin-induced nephrotoxicity. PLoS One 15, e0238816 (2020).
Deng, J. et al. Interleukin-10 inhibits ischemic and cisplatin-induced acute renal injury. Kidney Int. 60, 2118–2128 (2001).
Ma, Z. et al. Single-nucleus transcriptional profiling of chronic kidney disease after cisplatin nephrotoxicity. Am. J. Pathol. 192, 613–628 (2022).
Schett, G. & Neurath, M. F. Resolution of chronic inflammatory disease: universal and tissue-specific concepts. Nat. Commun. 9, 3261 (2018).
Guo, C. et al. DNA methylation protects against cisplatin-induced kidney injury by regulating specific genes, including interferon regulatory factor 8. Kidney Int. 92, 1194–1205 (2017).
Bao, Y. et al. DNA demethylase Tet2 suppresses cisplatin-induced acute kidney injury. Cell Death Discov. 7, 167 (2021).
Yu, Z. et al. Vitamin C deficiency causes cell type-specific epigenetic reprogramming and acute tubular necrosis in a mouse model. J. Am. Soc. Nephrol. 33, 531–546 (2022).
Jiang, X. et al. The role of m6A modification in the biological functions and diseases. Signal. Transduct. Target. Ther. 6, 74 (2021).
Li, C. M., Li, M., Zhao, W. B., Ye, Z. C. & Peng, H. Alteration of N6-methyladenosine RNA profiles in cisplatin-induced acute kidney injury in mice. Front. Mol. Biosci. 8, 654465 (2021).
Shen, J. et al. Integrated analysis of m6A methylome in cisplatin-induced acute kidney injury and berberine alleviation in mouse. Front. Genet. 11, 584460 (2020).
Zhou, P., Wu, M., Ye, C., Xu, Q. & Wang, L. Meclofenamic acid promotes cisplatin-induced acute kidney injury by inhibiting fat mass and obesity-associated protein-mediated m6A abrogation in RNA. J. Biol. Chem. 294, 16908–16917 (2019).
Wang, J. N. et al. Inhibition of METTL3 attenuates renal injury and inflammation by alleviating TAB3 m6A modifications via IGF2BP2-dependent mechanisms. Sci. Transl. Med. 14, eabk2709 (2022).
Graff, J. & Tsai, L. H. Histone acetylation: molecular mnemonics on the chromatin. Nat. Rev. Neurosci. 14, 97–111 (2013).
Kim, J. Y., Jo, J., Leem, J. & Park, K. K. Inhibition of p300 by garcinol protects against cisplatin-induced acute kidney injury through suppression of oxidative stress, inflammation, and tubular cell death in mice. Antioxidants 9, 1271 (2020).
Sun, L., Liu, J., Yuan, Y., Zhang, X. & Dong, Z. Protective effect of the BET protein inhibitor JQ1 in cisplatin-induced nephrotoxicity. Am. J. Physiol. Renal Physiol. 315, F469–F478 (2018).
Dong, G., Luo, J., Kumar, V. & Dong, Z. Inhibitors of histone deacetylases suppress cisplatin-induced p53 activation and apoptosis in renal tubular cells. Am. J. Physiol. Renal Physiol. 298, F293–F300 (2010).
Zhu, H. et al. PSTPIP2 inhibits cisplatin-induced acute kidney injury by suppressing apoptosis of renal tubular epithelial cells. Cell Death Dis. 11, 1057 (2020).
Ranganathan, P. et al. Histone deacetylase-mediated silencing of AMWAP expression contributes to cisplatin nephrotoxicity. Kidney Int. 89, 317–326 (2016).
Mikami, D. et al. β-Hydroxybutyrate, a ketone body, reduces the cytotoxic effect of cisplatin via activation of HDAC5 in human renal cortical epithelial cells. Life Sci. 222, 125–132 (2019).
Tang, J. et al. Blockade of histone deacetylase 6 protects against cisplatin-induced acute kidney injury. Clin. Sci. 132, 339–359 (2018).
Kim, D. H. et al. SIRT1 activation by resveratrol ameliorates cisplatin-induced renal injury through deacetylation of p53. Am. J. Physiol. Renal Physiol. 301, F427–F435 (2011).
Kim, J. Y. et al. Pharmacological activation of Sirt1 ameliorates cisplatin-induced acute kidney injury by suppressing apoptosis, oxidative stress, and inflammation in mice. Antioxidants 8, 322 (2019).
Li, M. et al. Sirt3 modulates fatty acid oxidation and attenuates cisplatin-induced AKI in mice. J. Cell Mol. Med. 24, 5109–5121 (2020).
Li, Z. et al. Overexpressed SIRT6 attenuates cisplatin-induced acute kidney injury by inhibiting ERK1/2 signaling. Kidney Int. 93, 881–892 (2018).
Miyasato, Y. et al. Sirtuin 7 deficiency ameliorates cisplatin-induced acute kidney injury through regulation of the inflammatory response. Sci. Rep. 8, 5927 (2018).
Jung, Y. J., Park, W., Kang, K. P. & Kim, W. SIRT2 is involved in cisplatin-induced acute kidney injury through regulation of mitogen-activated protein kinase phosphatase-1. Nephrol. Dial. Transpl. 35, 1145–1156 (2020).
O’Brien, J., Hayder, H., Zayed, Y. & Peng, C. Overview of microRNA biogenesis, mechanisms of actions, and circulation. Front. Endocrinol. 9, 402 (2018).
de Godoy Torso, N. et al. Dysregulated microRNAs as biomarkers or therapeutic targets in cisplatin-induced nephrotoxicity: a systematic review. Int. J. Mol. Sci. 22, 12765 (2021).
Bhatt, K. et al. MicroRNA-34a is induced via p53 during cisplatin nephrotoxicity and contributes to cell survival. Mol. Med. 16, 409–416 (2010).
Guo, Y. et al. MicroRNA-709 mediates acute tubular injury through effects on mitochondrial function. J. Am. Soc. Nephrol. 29, 449–461 (2018).
Hao, J. et al. MicroRNA-375 is induced in cisplatin nephrotoxicity to repress hepatocyte nuclear factor 1-β. J. Biol. Chem. 292, 4571–4582 (2017).
Lee, C. G. et al. Discovery of an integrative network of microRNAs and transcriptomics changes for acute kidney injury. Kidney Int. 86, 943–953 (2014).
Pellegrini, K. L. et al. MicroRNA-155 deficient mice experience heightened kidney toxicity when dosed with cisplatin. Toxicol. Sci. 141, 484–492 (2014).
Liao, W. et al. MicroRNA-140-5p attenuated oxidative stress in Cisplatin induced acute kidney injury by activating Nrf2/ARE pathway through a Keap1-independent mechanism. Exp. Cell Res. 360, 292–302 (2017).
Du, B. et al. MiR-30c regulates cisplatin-induced apoptosis of renal tubular epithelial cells by targeting Bnip3L and Hspa5. Cell Death Dis. 8, e2987 (2017).
Statello, L., Guo, C. J., Chen, L. L. & Huarte, M. Gene regulation by long non-coding RNAs and its biological functions. Nat. Rev. Mol. Cell Biol. 22, 96–118 (2021).
Zhang, Y. et al. Long non-coding RNA LRNA9884 promotes acute kidney injury via regulating NF-kB-mediated transcriptional activation of MIF. Front. Physiol. 11, 590027 (2020).
Chang, S. et al. LncRNA OIP5-AS1 reduces renal epithelial cell apoptosis in cisplatin-induced AKI by regulating the miR-144-5p/PKM2 axis. Biomed. J. https://doi.org/10.1016/j.bj.2021.07.005 (2021).
Li, J., Fan, X., Wang, Q., Gong, Y. & Guo, L. Long noncoding RNA PRNCR1 reduces renal epithelial cell apoptosis in cisplatin-induced AKI by regulating miR-182-5p/EZH1. Kidney Blood Press. Res. 46, 162–172 (2021).
Zhou, X. et al. Novel lncRNA XLOC_032768 alleviates cisplatin-induced apoptosis and inflammatory response of renal tubular epithelial cells through TNF-α. Int. Immunopharmacol. 83, 106472 (2020).
Greene, J. et al. Circular RNAs: biogenesis, function and role in human diseases. Front. Mol. Biosci. 4, 38 (2017).
Cao, Y. et al. Transcriptome sequencing of circular RNA reveals a novel circular RNA-has_circ_0114427 in the regulation of inflammation in acute kidney injury. Clin. Sci. 134, 139–154 (2020).
Li, C. M. et al. Circular RNA expression profiles in cisplatin-induced acute kidney injury in mice. Epigenomics 11, 1191–1207 (2019).
Wu, L. et al. Bone marrow mesenchymal stem cells ameliorate cisplatin-induced renal fibrosis via miR-146a-5p/Tfdp2 axis in renal tubular epithelial cells. Front. Immunol. 11, 623693 (2020).
Zarjou, A. et al. Paracrine effects of mesenchymal stem cells in cisplatin-induced renal injury require heme oxygenase-1. Am. J. Physiol. Renal Physiol. 300, F254–F262 (2011).
Zhang, R. et al. Resveratrol improves human umbilical cord-derived mesenchymal stem cells repair for cisplatin-induced acute kidney injury. Cell Death Dis. 9, 965 (2018).
Huang, X., Ma, Y., Li, Y., Han, F. & Lin, W. Targeted drug delivery systems for kidney diseases. Front. Bioeng. Biotechnol. 9, 683247 (2021).
Weng, Q. et al. Catalytic activity tunable ceria nanoparticles prevent chemotherapy-induced acute kidney injury without interference with chemotherapeutics. Nat. Commun. 12, 1436 (2021).
Moreno-Gordaliza, E., Marazuela, M. D., Pastor, O., Lazaro, A. & Gomez-Gomez, M. M. Lipidomics reveals cisplatin-induced renal lipid alterations during acute kidney injury and their attenuation by cilastatin. Int. J. Mol. Sci. 22, 12521 (2021).
Spath, M. R. et al. The proteome microenvironment determines the protective effect of preconditioning in cisplatin-induced acute kidney injury. Kidney Int. 95, 333–349 (2019).
Wu, R. et al. Comprehensive molecular and cellular characterization of acute kidney injury progression to renal fibrosis. Front. Immunol. 12, 699192 (2021).
Joo, M. S., Lee, C. G., Koo, J. H. & Kim, S. G. miR-125b transcriptionally increased by Nrf2 inhibits AhR repressor, which protects kidney from cisplatin-induced injury. Cell Death Dis. 4, e899 (2013).
Liu, H. & Baliga, R. Cytochrome P450 2E1 null mice provide novel protection against cisplatin-induced nephrotoxicity and apoptosis. Kidney Int. 63, 1687–1696 (2003).
Sahu, B. D., Mahesh Kumar, J. & Sistla, R. Baicalein, a bioflavonoid, prevents cisplatin-induced acute kidney injury by up-regulating antioxidant defenses and down-regulating the MAPKs and NF-κB pathways. PLoS One 10, e0134139 (2015).
Acknowledgements
The authors’ work was supported in part by grants from the National Natural Science Foundation of China (81870474), the National Institutes of Health (DK058831, DK087843) and the US Department of Veterans Affairs (BX000319). Z.D. is a recipient of the Senior Research Career Scientist award from the US Department of Veterans Affairs.
Author information
Authors and Affiliations
Contributions
C.T. and Z.D. researched data for the article and wrote the text. All authors contributed substantially to discussion of the content and reviewed and/or edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Nephrology thanks W. Brian Reeves, Aihua Zhang and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Glossary
- Mitophagy
-
A cellular process that selectively removes damaged or obsolete mitochondria via the autophagy–lysosome pathway.
- Ferritinophagy
-
A selective form of autophagy in which nuclear receptor coactivator 4 selectively binds to and delivers iron-laden ferritin to autophagosomes, leading to ferritin degradation in lysosomes and the release of free irons into the cytosol.
- Pattern recognition receptors
-
Receptors that recognize molecules from pathogens or released by damaged cells and subsequently trigger intracellular signalling cascades to induce transcriptional expression of inflammatory mediators that coordinate the elimination of pathogens and infected cells.
Rights and permissions
About this article
Cite this article
Tang, C., Livingston, M.J., Safirstein, R. et al. Cisplatin nephrotoxicity: new insights and therapeutic implications. Nat Rev Nephrol 19, 53–72 (2023). https://doi.org/10.1038/s41581-022-00631-7
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41581-022-00631-7
This article is cited by
-
Dihydroartemisinin abolishes cisplatin-induced nephrotoxicity in vivo
Journal of Natural Medicines (2024)
-
m6A-regulated tumor glycolysis: new advances in epigenetics and metabolism
Molecular Cancer (2023)
-
Regulated cell death pathways in kidney disease
Nature Reviews Nephrology (2023)
-
MicroRNA-mediated attenuation of branched-chain amino acid catabolism promotes ferroptosis in chronic kidney disease
Nature Communications (2023)
-
Ammonium tetrathiomolybdate relieves oxidative stress in cisplatin-induced acute kidney injury via NRF2 signaling pathway
Cell Death Discovery (2023)