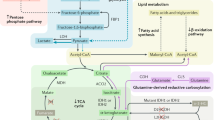
Abstract
Kidney cancer is the seventh leading cause of cancer in the world, and its incidence is on the rise. Renal cell carcinoma (RCC) is the most common form and is a heterogeneous disease comprising three major subtypes that vary in their histology, clinical course and driver mutations. These subtypes include clear cell RCC, papillary RCC and chromophobe RCC. Molecular analyses of hereditary and sporadic forms of RCC have revealed that this complex and deadly disease is characterized by metabolic pathway alterations in cancer cells that lead to deregulated oxygen and nutrient sensing, as well as impaired tricarboxylic acid cycle activity. These metabolic changes facilitate tumour growth and survival. Specifically, studies of the metabolic features of RCC have led to the discovery of oncometabolites — fumarate and succinate — that can promote tumorigenesis, moonlighting functions of enzymes, and substrate auxotrophy owing to the disruption of pathways that enable the production of arginine and cholesterol. These metabolic alterations within RCC can be exploited to identify new therapeutic targets and interventions, in combination with novel approaches that minimize the systemic toxicity of metabolic inhibitors and reduce the risk of drug resistance owing to metabolic plasticity.
Key points
-
Renal cell carcinoma (RCC) is a metabolic disease that develops from mutations in genes essential for cellular metabolism.
-
Metabolic alterations and adaptations allow cancer cells to proliferate, survive nutrient depletion and hypoxia, as well as promoting immune evasion, metastasis and resistance to therapy.
-
Hereditary RCC syndromes have demonstrated how perturbations in nutrient and oxygen sensing pathways, and the tricarboxylic acid cycle drive RCC tumorigenesis.
-
Sporadic RCC subtypes have both unique and shared metabolic alterations that promote cancer progression.
-
A lack of preclinical models for non-clear cell RCC tumours has limited the study of these important diseases.
-
Clarification of RCC-associated metabolic alterations can provide novel therapeutic targets, and metabolic interventions for RCC are currently in development.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Siegel, R. L., Miller, K. D., Wagle, N. S. & Jemal, A. Cancer statistics, 2023. CA Cancer J. Clin. 73, 17–48 (2023).
Kalra, S. et al. Prognosis of patients with metastatic renal cell carcinoma and pancreatic metastases. BJU Int. 117, 761–765 (2016).
Riscal, R. et al. Cholesterol auxotrophy as a targetable vulnerability in clear cell renal cell carcinoma. Cancer Discov. 11, 3106–3125 (2021).
Pavlova, N. N. & Thompson, C. B. The emerging hallmarks of cancer metabolism. Cell Metab. 23, 27–47 (2016).
Jang, C., Chen, L. & Rabinowitz, J. D. Metabolomics and isotope tracing. Cell 173, 822–837 (2018).
Dang, L. et al. Cancer-associated IDH1 mutations produce 2-hydroxyglutarate. Nature 462, 739–744 (2009).
Vander Heiden, M. G., Cantley, L. C. & Thompson, C. B. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science 324, 1029–1033 (2009).
Wang, Y. et al. Saturation of the mitochondrial NADH shuttles drives aerobic glycolysis in proliferating cells. Mol. Cell 82, 3270–3283.e3279 (2022).
Luengo, A. et al. Increased demand for NAD(+) relative to ATP drives aerobic glycolysis. Mol. Cell 81, 691–707.e696 (2021).
Maher, E. R. et al. Clinical features and natural history of von Hippel-Lindau disease. Q. J. Med. 77, 1151–1163 (1990).
Beroukhim, R. et al. Patterns of gene expression and copy-number alterations in Von-Hippel Lindau disease-associated and sporadic clear cell carcinoma of the kidney. Cancer Res. 69, 4674–4681 (2009).
Maxwell, P. H. et al. The tumour suppressor protein VHL targets hypoxia-inducible factors for oxygen-dependent proteolysis. Nature 399, 271–275 (1999).
Wang, G. L., Jiang, B. H., Rue, E. A. & Semenza, G. L. Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS heterodimer regulated by cellular O2 tension. Proc. Natl Acad. Sci. USA 92, 5510–5514 (1995).
Ortiz-Barahona, A., Villar, D., Pescador, N., Amigo, J. & del Peso, L. Genome-wide identification of hypoxia-inducible factor binding sites and target genes by a probabilistic model integrating transcription-profiling data and in silico binding site prediction. Nucleic Acids Res. 38, 2332–2345 (2010).
Smythies, J. A. et al. Inherent DNA-binding specificities of the HIF-1α and HIF-2α transcription factors in chromatin. EMBO Rep. 20, e46401 (2019).
Taylor, C. T. & Scholz, C. C. The effect of HIF on metabolism and immunity. Nat. Rev. Nephrol. 18, 573–587 (2022).
Iyer, N. V. et al. Cellular and developmental control of O2 homeostasis by hypoxia-inducible factor 1α. Genes. Dev. 12, 149–162 (1998).
Forsythe, J. A. et al. Activation of vascular endothelial growth factor gene transcription by hypoxia-inducible factor 1. Mol. Cell. Biol. 16, 4604–4613 (1996).
Kelly, B. D. et al. Cell type-specific regulation of angiogenic growth factor gene expression and induction of angiogenesis in nonischemic tissue by a constitutively active form of hypoxia-inducible factor 1. Circ. Res. 93, 1074–1081 (2003).
Motzer, R. J. et al. Overall survival and updated results for sunitinib compared with interferon alfa in patients with metastatic renal cell carcinoma. J. Clin. Oncol. 27, 3584–3590 (2009).
Kim, J. W., Tchernyshyov, I., Semenza, G. L. & Dang, C. V. HIF-1-mediated expression of pyruvate dehydrogenase kinase: a metabolic switch required for cellular adaptation to hypoxia. Cell Metab. 3, 177–185 (2006).
Semenza, G. L. et al. Hypoxia response elements in the aldolase A, enolase 1, and lactate dehydrogenase A gene promoters contain essential binding sites for hypoxia-inducible factor 1. J. Biol. Chem. 271, 32529–32537 (1996).
Ullah, M. S., Davies, A. J. & Halestrap, A. P. The plasma membrane lactate transporter MCT4, but not MCT1, is up-regulated by hypoxia through a HIF-1alpha-dependent mechanism. J. Biol. Chem. 281, 9030–9037 (2006).
Xu, R. et al. 3-[(1S,2S,3R)-2,3-Difluoro-1-hydroxy-7-methylsulfonylindan-4-yl]oxy-5-fluorobenzonitrile (PT2977), a Hypoxia-Inducible Factor 2α (HIF-2α) inhibitor for the treatment of clear cell renal cell carcinoma. J. Med. Chem. 62, 6876–6893 (2019).
Chen, W. et al. Targeting renal cell carcinoma with a HIF-2 antagonist. Nature 539, 112–117 (2016).
Wehn, P. M. et al. Design and activity of specific hypoxia-inducible factor-2α (HIF-2α) inhibitors for the treatment of clear cell renal cell carcinoma: discovery of clinical candidate (S)-3-((2,2-Difluoro-1-hydroxy-7-(methylsulfonyl)-2,3-dihydro-1H-inden-4-yl)oxy)-5-fluorobenzonitrile (PT2385). J. Med. Chem. 61, 9691–9721 (2018).
Jonasch, E. et al. Belzutifan for renal cell carcinoma in Von Hippel–Lindau disease. N. Engl. J. Med. 385, 2036–2046 (2021).
Courtney, K. D. et al. Phase I dose-escalation trial of PT2385, a first-in-class hypoxia-inducible factor-2α antagonist in patients with previously treated advanced clear cell renal cell carcinoma. J. Clin. Oncol. 36, 867–874 (2018).
Stransky, L. A. et al. Sensitivity of VHL mutant kidney cancers to HIF2 inhibitors does not require an intact p53 pathway. Proc. Natl Acad. Sci. USA 119, e2120403119 (2022).
Courtney, K. D. et al. HIF-2 complex dissociation, target inhibition, and acquired resistance with PT2385, a first-in-class HIF-2 inhibitor, in patients with clear cell renal cell carcinoma. Clin. Cancer Res. 26, 793–803 (2020).
Andreou, A. et al. Elongin C (ELOC/TCEB1)-associated von Hippel-Lindau disease. Hum. Mol. Genet. 31, 2728–2737 (2022).
Hakimi, A. A. et al. TCEB1-mutated renal cell carcinoma: a distinct genomic and morphological subtype. Mod. Pathol. 28, 845–853 (2015).
Sato, Y. et al. Integrated molecular analysis of clear-cell renal cell carcinoma. Nat. Genet. 45, 860–867 (2013).
Kandoth, C. et al. Mutational landscape and significance across 12 major cancer types. Nature 502, 333–339 (2013).
Inoki, K., Li, Y., Zhu, T., Wu, J. & Guan, K.-L. TSC2 is phosphorylated and inhibited by Akt and suppresses mTOR signalling. Nat. Cell Biol. 4, 648–657 (2002).
Ma, X. M. & Blenis, J. Molecular mechanisms of mTOR-mediated translational control. Nat. Rev. Mol. Cell Biol. 10, 307–318 (2009).
Laplante, M. & Sabatini, D. M. mTOR signaling in growth control and disease. Cell 149, 274–293 (2012).
Schmidt, L. et al. Germline and somatic mutations in the tyrosine kinase domain of the MET proto-oncogene in papillary renal carcinomas. Nat. Genet. 16, 68–73 (1997).
Maher, E. R. Hereditary renal cell carcinoma syndromes: diagnosis, surveillance and management. World J. Urol. 36, 1891–1898 (2018).
Linehan, W. M. et al. Comprehensive molecular characterization of papillary renal-cell carcinoma. N. Engl. J. Med. 374, 135–145 (2016).
Yin, X. et al. Relationships between chromosome 7 gain, MET gene copy number increase and MET protein overexpression in Chinese papillary renal cell carcinoma patients. PLoS ONE 10, e0143468 (2015).
Boccaccio, C. et al. Induction of epithelial tubules by growth factor HGF depends on the STAT pathway. Nature 391, 285–288 (1998).
Ponzetto, C. et al. A novel recognition motif for phosphatidylinositol 3-kinase binding mediates its association with the hepatocyte growth factor/scatter factor receptor. Mol. Cell Biol. 13, 4600–4608 (1993).
Graziani, A., Gramaglia, D., dalla Zonca, P. & Comoglio, P. M. Hepatocyte growth factor/scatter factor stimulates the Ras-guanine nucleotide exchanger. J. Biol. Chem. 268, 9165–9168 (1993).
Choueiri, T. K. et al. Phase II and biomarker study of the dual MET/VEGFR2 inhibitor foretinib in patients with papillary renal cell carcinoma. J. Clin. Oncol. 31, 181–186 (2013).
Kim, R. H. et al. Early-onset renal cell carcinoma in PTEN harmatoma tumour syndrome. NPJ Genom. Med. 5, 40 (2020).
Tibarewal, P. et al. Long-term treatment of cancer-prone germline PTEN mutant mice with low-dose rapamycin extends lifespan and delays tumour development. J. Pathol. 258, 382–394 (2022).
Squarize, C. H., Castilho, R. M. & Gutkind, J. S. Chemoprevention and treatment of experimental Cowden’s disease by mTOR inhibition with rapamycin. Cancer Res. 68, 7066–7072 (2008).
Komiya, T. et al. A pilot study of sirolimus in subjects with Cowden syndrome or other syndromes characterized by germline mutations in PTEN. Oncologist 24, 1510–e1265 (2019).
Rodon, J. et al. Phase 1/1b dose escalation and expansion study of BEZ235, a dual PI3K/mTOR inhibitor, in patients with advanced solid tumors including patients with advanced breast cancer. Cancer Chemother. Pharmacol. 82, 285–298 (2018).
Yang, P. et al. Renal cell carcinoma in tuberous sclerosis complex. Am. J. Surg. Pathol. 38, 895–909 (2014).
Bissler, J. J. et al. Sirolimus for angiomyolipoma in tuberous sclerosis complex or lymphangioleiomyomatosis. N. Engl. J. Med. 358, 140–151 (2008).
Maroto, P. et al. Biallelic TSC2 mutations in a patient with chromophobe renal cell carcinoma showing extraordinary response to temsirolimus. J. Natl Compr. Canc Netw. 16, 352–358 (2018).
Nickerson, M. L. et al. Mutations in a novel gene lead to kidney tumors, lung wall defects, and benign tumors of the hair follicle in patients with the Birt-Hogg-Dubé syndrome. Cancer Cell 2, 157–164 (2002).
Preston, R. S. et al. Absence of the Birt-Hogg-Dubé gene product is associated with increased hypoxia-inducible factor transcriptional activity and a loss of metabolic flexibility. Oncogene 30, 1159–1173 (2011).
Hasumi, H. et al. Identification and characterization of a novel folliculin-interacting protein FNIP2. Gene 415, 60–67 (2008).
Baba, M. et al. Folliculin encoded by the BHD gene interacts with a binding protein, FNIP1, and AMPK, and is involved in AMPK and mTOR signaling. Proc. Natl Acad. Sci. USA 103, 15552–15557 (2006).
Lawrence, R. E. et al. Structural mechanism of a Rag GTPase activation checkpoint by the lysosomal folliculin complex. Science 366, 971–977 (2019).
Shen, K. et al. Cryo-EM structure of the human FLCN-FNIP2-Rag-ragulator complex. Cell 179, 1319–1329.e1318 (2019).
Chen, J. et al. Deficiency of FLCN in mouse kidney led to development of polycystic kidneys and renal neoplasia. PLoS ONE 3, e3581 (2008).
Napolitano, G. et al. A substrate-specific mTORC1 pathway underlies Birt–Hogg–Dubé syndrome. Nature 585, 597–602 (2020).
Baba, M. et al. Kidney-targeted Birt-Hogg-Dube gene inactivation in a mouse model: Erk1/2 and Akt-mTOR activation, cell hyperproliferation, and polycystic kidneys. J. Natl Cancer Inst. 100, 140–154 (2008).
Wu, M. et al. Flcn-deficient renal cells are tumorigenic and sensitive to mTOR suppression. Oncotarget 6, 32761–32773 (2015).
ClinicalTrials.gov. US National Library of Medicine. https://clinicaltrials.gov/study/NCT02504892 (2019).
Laviolette, L. A. et al. Negative regulation of EGFR signalling by the human folliculin tumour suppressor protein. Nat. Commun. 8, 15866 (2017).
Hasumi, H. et al. BHD-associated kidney cancer exhibits unique molecular characteristics and a wide variety of variants in chromatin remodeling genes. Hum. Mol. Genet. 27, 2712–2724 (2018).
Klomp, J. A. et al. Birt-Hogg-Dubé renal tumors are genetically distinct from other renal neoplasias and are associated with up-regulation of mitochondrial gene expression. BMC Med. Genomics 3, 59 (2010).
Bertolotto, C. et al. A SUMOylation-defective MITF germline mutation predisposes to melanoma and renal carcinoma. Nature 480, 94–98 (2011).
Sun, G. et al. Integrated exome and RNA sequencing of TFE3-translocation renal cell carcinoma. Nat. Commun. 12, 5262 (2021).
Perlman, E. J. Pediatric renal cell carcinoma. Surgical Pathol. Clin. 3, 641–651 (2010).
Sardiello, M. et al. A gene network regulating lysosomal biogenesis and function. Science 325, 473–477 (2009).
Settembre, C. et al. TFEB links autophagy to lysosomal biogenesis. Science 332, 1429–1433 (2011).
Martina, J. A. et al. The nutrient-responsive transcription factor TFE3 promotes autophagy, lysosomal biogenesis, and clearance of cellular debris. Sci. Signal. 7, ra9 (2014).
Damayanti, N. P. et al. Therapeutic targeting of TFE3/IRS-1/PI3K/mTOR axis in translocation renal cell carcinoma. Clin. Cancer Res. 24, 5977–5989 (2018).
Vanharanta, S. et al. Early-onset renal cell carcinoma as a novel extraparaganglial component of SDHB-associated heritable paraganglioma. Am. J. Hum. Genet. 74, 153–159 (2004).
Lehtonen, R. et al. Biallelic inactivation of fumarate hydratase (FH) occurs in nonsyndromic uterine leiomyomas but is rare in other tumors. Am. J. Pathol. 164, 17–22 (2004).
Hewitson, K. S. et al. Structural and mechanistic studies on the inhibition of the hypoxia-inducible transcription factor hydroxylases by tricarboxylic acid cycle intermediates. J. Biol. Chem. 282, 3293–3301 (2007).
Isaacs, J. S. et al. HIF overexpression correlates with biallelic loss of fumarate hydratase in renal cancer: novel role of fumarate in regulation of HIF stability. Cancer Cell 8, 143–153 (2005).
Selak, M. A. et al. Succinate links TCA cycle dysfunction to oncogenesis by inhibiting HIF-alpha prolyl hydroxylase. Cancer Cell 7, 77–85 (2005).
Xiao, M. et al. Inhibition of α-KG-dependent histone and DNA demethylases by fumarate and succinate that are accumulated in mutations of FH and SDH tumor suppressors. Genes. Dev. 26, 1326–1338 (2012).
Smith, E. H., Janknecht, R. & Maher, L. J. III Succinate inhibition of α-ketoglutarate-dependent enzymes in a yeast model of paraganglioma. Hum. Mol. Genet. 16, 3136–3148 (2007).
Ricketts, C. J. et al. Kidney tumors associated with germline mutations of FH and SDHB show a CpG island methylator phenotype (CIMP). PLoS ONE 17, e0278108 (2022).
Sciacovelli, M. et al. Fumarate is an epigenetic modifier that elicits epithelial-to-mesenchymal transition. Nature 537, 544–547 (2016).
Ligon, J. A. et al. A phase II trial of guadecitabine in children and adults with SDH-deficient GIST, pheochromocytoma, paraganglioma, and HLRCC-associated renal cell carcinoma. Clin. Cancer Res. 29, 341–348 (2023).
Sulkowski, P. L. et al. Krebs-cycle-deficient hereditary cancer syndromes are defined by defects in homologous-recombination DNA repair. Nat. Genet. 50, 1086–1092 (2018).
Ueno, D. et al. Targeting Krebs-cycle-deficient renal cell carcinoma with Poly ADP-ribose polymerase inhibitors and low-dose alkylating chemotherapy. Oncotarget 13, 1054–1067 (2022).
Tong, W. H. et al. The glycolytic shift in fumarate-hydratase-deficient kidney cancer lowers AMPK levels, increases anabolic propensities and lowers cellular iron levels. Cancer Cell 20, 315–327 (2011).
Mullen, A. R. et al. Reductive carboxylation supports growth in tumour cells with defective mitochondria. Nature 481, 385–388 (2011).
Frezza, C. et al. Haem oxygenase is synthetically lethal with the tumour suppressor fumarate hydratase. Nature 477, 225–228 (2011).
Yoo, A. et al. Genomic and metabolic hallmarks of SDH- and FH-deficient renal cell carcinomas. Eur. Urol. Focus. 8, 1278–1288 (2022).
Zheng, L. et al. Reversed argininosuccinate lyase activity in fumarate hydratase-deficient cancer cells. Cancer Metab. 1, 12 (2013).
Alderson, N. L. et al. S-(2-Succinyl)cysteine: a novel chemical modification of tissue proteins by a Krebs cycle intermediate. Arch. Biochem. Biophys. 450, 1–8 (2006).
Ternette, N. et al. Inhibition of mitochondrial aconitase by succination in fumarate hydratase deficiency. Cell Rep. 3, 689–700 (2013).
Bardella, C. et al. Aberrant succination of proteins in fumarate hydratase-deficient mice and HLRCC patients is a robust biomarker of mutation status. J. Pathol. 225, 4–11 (2011).
Adam, J. et al. Renal cyst formation in Fh1-deficient mice is independent of the Hif/Phd pathway: roles for fumarate in KEAP1 succination and Nrf2 signaling. Cancer Cell 20, 524–537 (2011).
Kulkarni, R. A. et al. A chemoproteomic portrait of the oncometabolite fumarate. Nat. Chem. Biol. 15, 391–400 (2019).
Creighton, C. J. et al. Comprehensive molecular characterization of clear cell renal cell carcinoma. Nature 499, 43–49 (2013).
Shapiro, D. D., Virumbrales-Muñoz, M., Beebe, D. J. & Abel, E. J. Models of renal cell carcinoma used to investigate molecular mechanisms and develop new therapeutics. Front. Oncol. 12, 871252 (2022).
Lobo, J. et al. WHO 2022 landscape of papillary and chromophobe renal cell carcinoma. Histopathology 81, 426–438 (2022).
Pan, H., Ye, L., Zhu, Q., Yang, Z. & Hu, M. The effect of the papillary renal cell carcinoma subtype on oncological outcomes. Sci. Rep. 10, 21073 (2020).
Wang, Q. et al. Single-cell chromatin accessibility landscape in kidney identifies additional cell-of-origin in heterogenous papillary renal cell carcinoma. Nat. Commun. 13, 31 (2022).
Albiges, L. et al. MET is a potential target across all papillary renal cell carcinomas: result from a large molecular study of pRCC with CGH array and matching gene expression array. Clin. Cancer Res. 20, 3411–3421 (2014).
Schuller, A. G. et al. The MET inhibitor AZD6094 (Savolitinib, HMPL-504) induces regression in papillary renal cell carcinoma patient–derived xenograft models. Clin. Cancer Res. 21, 2811–2819 (2015).
Chawla, N. S. et al. An update on the treatment of papillary renal cell carcinoma. Cancers 15, 565 (2023).
Pal, S. K. et al. A comparison of sunitinib with cabozantinib, crizotinib, and savolitinib for treatment of advanced papillary renal cell carcinoma: a randomised, open-label, phase 2 trial. Lancet 397, 695–703 (2021).
Moch, H. et al. The 2022 World Health organization classification of tumours of the urinary system and male genital organs — part A: renal, penile, and testicular tumours. Eur. Urol. 82, 458–468 (2022).
Ahmad, A. A. et al. Papillary renal cell carcinomas rewire glutathione metabolism and are deficient in both anabolic glucose synthesis and oxidative phosphorylation. Cancers 11, 1298 (2019).
Sun, C. et al. Overexpression of enolase 2 is associated with worsened prognosis and increased glycikolysis in papillary renal cell carcinoma. J. Cell. Physiol. 236, 3821–3831 (2021).
Nakajima, R. et al. Evaluation of renal cell carcinoma histological subtype and Fuhrman grade using 18F-fluorodeoxyglucose-positron emission tomography/computed tomography. Eur. Radiol. 27, 4866–4873 (2017).
Ricketts, C. J. et al. The Cancer Genome Atlas comprehensive molecular characterization of renal cell carcinoma. Cell Rep. 23, 313–326.e5 (2018).
Kaushik, A. K. et al. In vivo characterization of glutamine metabolism identifies therapeutic targets in clear cell renal cell carcinoma. Sci. Adv. 8, eabp8293 (2022).
Volpe, A. et al. Chromophobe renal cell carcinoma (RCC): oncological outcomes and prognostic factors in a large multicentre series. BJU Int. 110, 76–83 (2012).
Davis, C. F. et al. The somatic genomic landscape of chromophobe renal cell carcinoma. Cancer Cell 26, 319–330 (2014).
Durinck, S. et al. Spectrum of diverse genomic alterations define non-clear cell renal carcinoma subtypes. Nat. Genet. 47, 13–21 (2015).
Roldan-Romero, J. M. et al. Molecular characterization of chromophobe renal cell carcinoma reveals mTOR pathway alterations in patients with poor outcome. Mod. Pathol. 33, 2580–2590 (2020).
Casuscelli, J. et al. Genomic landscape and evolution of metastatic chromophobe renal cell carcinoma. JCI Insight 2, e92688 (2017).
Garje, R. et al. Comprehensive review of chromophobe renal cell carcinoma. Crit. Rev. Oncol. Hematol. 160, 103287 (2021).
Tickoo, S. K. et al. Ultrastructural observations on mitochondria and microvesicles in renal oncocytoma, chromophobe renal cell carcinoma, and eosinophilic variant of conventional (clear cell) renal cell carcinoma. Am. J. Surg. Pathol. 24, 1247–1256 (2000).
Divya, B. et al. Mitochondrial metabolism in primary and metastatic human kidney cancers. Preprint at bioRxiv, https://doi.org/10.1101/2023.02.06.527285 (2023).
Schaeffeler, E. et al. Metabolic and lipidomic reprogramming in renal cell carcinoma subtypes reflects regions of tumor origin. Eur. Urol. Focus. 5, 608–618 (2019).
Xiao, Y. et al. Endocytosis-mediated replenishment of amino acids favors cancer cell proliferation and survival in chromophobe renal cell carcinoma. Cancer Res. 80, 5491–5501 (2020).
Priolo, C. et al. Impairment of gamma-glutamyl transferase 1 activity in the metabolic pathogenesis of chromophobe renal cell carcinoma. Proc. Natl Acad. Sci. USA 115, E6274–E6282 (2018).
Zhang, L. et al. Hypersensitivity to ferroptosis in chromophobe RCC is mediated by a glutathione metabolic dependency and cystine import via solute carrier family 7 member 11. Proc. Natl Acad. Sci. USA 119, e2122840119 (2022).
Simon, A. G. et al. Targeting glycolysis with 2-deoxy-D-glucose sensitizes primary cell cultures of renal cell carcinoma to tyrosine kinase inhibitors. J. Cancer Res. Clin. Oncol. 146, 2255–2265 (2020).
Weiss, R. H. Metabolomics and metabolic reprogramming in kidney cancer. Semin. Nephrol. 38, 175–182 (2018).
Linehan, W. M., Srinivasan, R. & Schmidt, L. S. The genetic basis of kidney cancer: a metabolic disease. Nat. Rev. Urol. 7, 277–285 (2010).
Linehan, W. M. & Ricketts, C. J. The metabolic basis of kidney cancer. Semin. Cancer Biol. 23, 46–55 (2013).
Hakimi, A. A. et al. An integrated metabolic atlas of clear cell renal cell carcinoma. Cancer Cell 29, 104–116 (2016).
Clark, D. J. et al. Integrated proteogenomic characterization of clear cell renal cell carcinoma. Cell 179, 964–983.e931 (2019).
Lucarelli, G. et al. Metabolomic profile of glycolysis and the pentose phosphate pathway identifies the central role of glucose-6-phosphate dehydrogenase in clear cell-renal cell carcinoma. Oncotarget 6, 13371–13386 (2015).
Wettersten, H. I. et al. Grade-dependent metabolic reprogramming in kidney cancer revealed by combined proteomics and metabolomics analysis. Cancer Res. 75, 2541–2552 (2015).
Courtney, K. D. et al. Isotope tracing of human clear cell renal cell carcinomas demonstrates suppressed glucose oxidation in vivo. Cell Metab. 28, 793–800.e792 (2018).
Ambrosetti, D. et al. The two glycolytic markers GLUT1 and MCT1 correlate with tumor grade and survival in clear-cell renal cell carcinoma. PLoS ONE 13, e0193477 (2018).
Girgis, H. et al. Lactate dehydrogenase a is a potential prognostic marker in clear cell renal cell carcinoma. Mol. Cancer 13, 101 (2014).
Kim, Y., Choi, J.-W., Lee, J.-H. & Kim, Y.-S. Expression of lactate/H+ symporters MCT1 and MCT4 and their chaperone CD147 predicts tumor progression in clear cell renal cell carcinoma: immunohistochemical and The Cancer Genome Atlas data analyses. Hum. Pathol. 46, 104–112 (2015).
Singer, K. et al. Warburg phenotype in renal cell carcinoma: high expression of glucose-transporter 1 (GLUT-1) correlates with low CD8+ T-cell infiltration in the tumor. Int. J. Cancer 128, 2085–2095 (2011).
Xu, W. et al. Hexokinase 3 dysfunction promotes tumorigenesis and immune escape by upregulating monocyte/macrophage infiltration into the clear cell renal cell carcinoma microenvironment. Int. J. Biol. Sci. 17, 2205–2222 (2021).
Reinfeld, B. I. et al. Cell-programmed nutrient partitioning in the tumour microenvironment. Nature 593, 282–288 (2021).
Quinn, W. J. III et al. Lactate limits T cell proliferation via the NAD(H) Redox State. Cell Rep. 33, 108500 (2020).
Bianchi, C. et al. The glucose and lipid metabolism reprogramming is grade-dependent in clear cell renal cell carcinoma primary cultures and is targetable to modulate cell viability and proliferation. Oncotarget 8, 113502–113515 (2017).
Nilsson, H. et al. Primary clear cell renal carcinoma cells display minimal mitochondrial respiratory capacity resulting in pronounced sensitivity to glycolytic inhibition by 3-bromopyruvate. Cell Death Dis. 6, e1585 (2015).
Zhang, Q. et al. Aerosolized 3-bromopyruvate inhibits lung tumorigenesis without causing liver toxicity. Cancer Prev. Res. 5, 717–725 (2012).
Wang, J. et al. The platelet isoform of phosphofructokinase contributes to metabolic reprogramming and maintains cell proliferation in clear cell renal cell carcinoma. Oncotarget 7 (2016).
Gerlinger, M. et al. Genome-wide RNA interference analysis of renal carcinoma survival regulators identifies MCT4 as a Warburg effect metabolic target. J. Pathol. 227, 146–156 (2012).
Goldberg, F. W. et al. Discovery of clinical candidate AZD0095, a selective inhibitor of monocarboxylate transporter 4 (MCT4) for oncology. J. Med. Chem. 66, 384–397 (2023).
Halford, S. et al. A phase I dose-escalation study of AZD3965, an oral monocarboxylate transporter 1 inhibitor, in patients with advanced cancer. Clin. Cancer Res. 29, 1429–1439 (2023).
Xu, W.-H. et al. Large-scale transcriptome profiles reveal robust 20-signatures metabolic prediction models and novel role of G6PC in clear cell renal cell carcinoma. J. Cell. Mol. Med. 24, 9012–9027 (2020).
Li, B. et al. Fructose-1,6-bisphosphatase opposes renal carcinoma progression. Nature 513, 251–255 (2014).
Shi, L., An, S., Liu, Y., Liu, J. & Wang, F. PCK1 regulates glycolysis and tumor progression in clear cell renal cell carcinoma through LDHA. Onco Targets Ther. 13, 2613–2627 (2020).
Godfrey, J., Riscal, R., Skuli, N. & Simon, M. C. Glucagon signaling via supraphysiologic GCGR can reduce cell viability without stimulating gluconeogenic gene expression in liver cancer cells. Cancer Metab. 10, 4 (2022).
Xie, H. et al. Glycogen metabolism is dispensable for tumour progression in clear cell renal cell carcinoma. Nat. Metab. 3, 327–336 (2021).
Safarinejad, M. R., Shafiei, N. & Safarinejad, S. Methylenetetrahydrofolate reductase (MTHFR) gene C677T, A1298C and G1793A polymorphisms: association with risk for clear cell renal cell carcinoma and tumour behaviour in men. Clin. Oncol. 24, 269–281 (2012).
Yoshino, H. et al. PHGDH as a key enzyme for serine biosynthesis in HIF2α-targeting therapy for renal cell carcinoma. Cancer Res. 77, 6321–6329 (2017).
Aggarwal, R. K. et al. Functional succinate dehydrogenase deficiency is a common adverse feature of clear cell renal cancer. Proc. Natl Acad. Sci. USA 118, e2106947118 (2021).
Ma, Y. et al. SIRT5-mediated SDHA desuccinylation promotes clear cell renal cell carcinoma tumorigenesis. Free Radic. Biol. Med. 134, 458–467 (2019).
Fang, Z., Sun, Q., Yang, H. & Zheng, J. SDHB suppresses the tumorigenesis and development of ccRCC by inhibiting glycolysis. Front. Oncol. 11, 639408 (2021).
Xu, X. et al. Upregulation of SDHA inhibited proliferation, migration, and invasion of clear cell renal cell carcinoma cells via inactivation of the Wnt/β-catenin pathway. J. Recept. Signal. Transduct. Res. 42, 180–188 (2022).
Ellinger, J. et al. Systematic expression analysis of the mitochondrial complex III subunits identifies UQCRC1 as biomarker in clear cell renal cell carcinoma. Oncotarget 7, 86490–86499 (2016).
Ellinger, J. et al. Systematic expression analysis of mitochondrial complex I identifies NDUFS1 as a biomarker in clear-cell renal-cell carcinoma. Clin. Genitourin. Cancer 15, e551–e562 (2017).
Brüggemann, M. et al. systematic analysis of the expression of the mitochondrial ATP synthase (Complex V) subunits in clear cell renal cell carcinoma. Transl. Oncol. 10, 661–668 (2017).
Luo, Y. et al. UQCRH downregulation promotes Warburg effect in renal cell carcinoma cells. Sci. Rep. 10, 15021 (2020).
Miyakuni, K. et al. Genome-wide analysis of DNA methylation identifies the apoptosis-related gene UQCRH as a tumor suppressor in renal cancer. Mol. Oncol. 16, 732–749 (2022).
Tello, D. et al. Induction of the mitochondrial NDUFA4L2 protein by HIF-1α decreases oxygen consumption by inhibiting complex I activity. Cell Metab. 14, 768–779 (2011).
Liu, L. et al. NDUFA4L2 expression predicts poor prognosis in clear cell renal cell carcinoma patients. Ren. Fail. 38, 1199–1205 (2016).
Lucarelli, G. et al. Integrated multi-omics characterization reveals a distinctive metabolic signature and the role of NDUFA4L2 in promoting angiogenesis, chemoresistance, and mitochondrial dysfunction in clear cell renal cell carcinoma. Aging 10, 3957–3985 (2018).
Minton, D. R. et al. Role of NADH dehydrogenase (Ubiquinone) 1 alpha subcomplex 4-like 2 in clear cell renal cell carcinoma. Clin. Cancer Res. 22, 2791–2801 (2016).
Kinnaird, A. et al. Metabolic modulation of clear-cell renal cell carcinoma with dichloroacetate, an inhibitor of pyruvate dehydrogenase kinase. Eur. Urol. 69, 734–744 (2016).
Ochocki, J. D. et al. Arginase 2 suppresses renal carcinoma progression via biosynthetic cofactor pyridoxal phosphate depletion and increased polyamine toxicity. Cell Metab. 27, 1263–1280.e6 (2018).
Khare, S. et al. ASS1 and ASL suppress growth in clear cell renal cell carcinoma via altered nitrogen metabolism. Cancer Metab. 9, 40 (2021).
Yoon, C. Y. et al. Renal cell carcinoma does not express argininosuccinate synthetase and is highly sensitive to arginine deprivation via arginine deiminase. Int. J. Cancer 120, 897–905 (2007).
Abu Aboud, O. et al. Glutamine addiction in kidney cancer suppresses oxidative stress and can be exploited for real-time imaging. Cancer Res. 77, 6746–6758 (2017).
Gameiro, P. A. et al. In vivo HIF-mediated reductive carboxylation is regulated by citrate levels and sensitizes VHL-deficient cells to glutamine deprivation. Cell Metab. 17, 372–385 (2013).
Biancur, D. E. et al. Compensatory metabolic networks in pancreatic cancers upon perturbation of glutamine metabolism. Nat. Commun. 8, 15965 (2017).
Meric-Bernstam, F. et al. Telaglenastat plus cabozantinib or everolimus for advanced or metastatic renal cell carcinoma: an open-label phase I trial. Clin. Cancer Res. 28, 1540–1548 (2022).
Lee, C.-H. et al. Telaglenastat plus everolimus in advanced renal cell carcinoma: a randomized, double-blinded, placebo-controlled, phase II ENTRATA trial. Clin. Cancer Res. 28, 3248–3255 (2022).
Tannir, N. M. et al. Efficacy and safety of telaglenastat plus cabozantinib vs placebo plus cabozantinib in patients with advanced renal cell carcinoma: the CANTATA randomized clinical trial. JAMA Oncol. 8, 1411–1418, (2022).
Thangavelu, K. et al. Structural basis for the allosteric inhibitory mechanism of human kidney-type glutaminase (KGA) and its regulation by Raf-Mek-Erk signaling in cancer cell metabolism. Proc. Natl Acad. Sci. USA 109, 7705–7710 (2012).
Lynch, G., Kemeny, N. & Casper, E. Phase II evaluation of DON (6-diazo-5-oxo-L-norleucine) in patients with advanced colorectal carcinoma. Am. J. Clin. Oncol. 5, 541–543 (1982).
Rais, R. et al. Discovery of DRP-104, a tumor-targeted metabolic inhibitor prodrug. Sci. Adv. 8, eabq5925 (2022).
ClinicalTrials.gov. US National Library of Medicine. https://clinicaltrials.gov/study/NCT04471415 (2023).
Bansal, A. et al. Gamma-glutamyltransferase 1 promotes clear cell renal cell carcinoma initiation and progression. Mol. Cancer Res. 17, 1881–1892 (2019).
Miess, H. et al. The glutathione redox system is essential to prevent ferroptosis caused by impaired lipid metabolism in clear cell renal cell carcinoma. Oncogene 37, 5435–5450 (2018).
Tang, X. et al. Cystine deprivation triggers programmed necrosis in VHL-deficient renal cell carcinomas. Cancer Res. 76, 1892–1903 (2016).
Zou, Y. et al. A GPX4-dependent cancer cell state underlies the clear-cell morphology and confers sensitivity to ferroptosis. Nat. Commun. 10, 1617 (2019).
Fujimoto, T. & Parton, R. G. Not just fat: the structure and function of the lipid droplet. Cold Spring Harb. Perspect. Biol. 3, a004838 (2011).
Tan, S. K., Hougen, H. Y., Merchan, J. R., Gonzalgo, M. L. & Welford, S. M. Fatty acid metabolism reprogramming in ccRCC: mechanisms and potential targets. Nat. Rev. Urol. 20, 48–60 (2023).
Qiu, B. et al. HIF2α-dependent lipid storage promotes endoplasmic reticulum homeostasis in clear-cell renal cell carcinoma. Cancer Discov. 5, 652–667 (2015).
Gebhard, R. L. et al. Abnormal cholesterol metabolism in renal clear cell carcinoma. J. Lipid Res. 28, 1177–1184 (1987).
Xu, G.-H. et al. Up-regulation of SR-BI promotes progression and serves as a prognostic biomarker in clear cell renal cell carcinoma. BMC Cancer 18, 88 (2018).
Rowe, I. A. et al. Effect of scavenger receptor class B type I antagonist ITX5061 in patients with hepatitis C virus infection undergoing liver transplantation. Liver Transpl. 22, 287–297 (2016).
Munn, D. H. et al. Prevention of allogeneic fetal rejection by tryptophan catabolism. Science 281, 1191–1193 (1998).
Platten, M., Nollen, E. A. A., Röhrig, U. F., Fallarino, F. & Opitz, C. A. Tryptophan metabolism as a common therapeutic target in cancer, neurodegeneration and beyond. Nat. Rev. Drug. Discov. 18, 379–401 (2019).
Frumento, G. et al. Tryptophan-derived catabolites are responsible for inhibition of T and natural killer cell proliferation induced by indoleamine 2,3-dioxygenase. J. Exp. Med. 196, 459–468 (2002).
Lucarelli, G. et al. Activation of the kynurenine pathway predicts poor outcome in patients with clear cell renal cell carcinoma. Urol. Oncol. 35, 461.e415–461.e427 (2017).
Riesenberg, R. et al. Expression of indoleamine 2,3-dioxygenase in tumor endothelial cells correlates with long-term survival of patients with renal cell carcinoma. Clin. Cancer Res. 13, 6993–7002 (2007).
Trott, J. F. et al. Inhibiting tryptophan metabolism enhances interferon therapy in kidney cancer. Oncotarget 7, 66540–66557 (2016).
Minasian, L. M. et al. Interferon alfa-2a in advanced renal cell carcinoma: treatment results and survival in 159 patients with long-term follow-up. J. Clin. Oncol. 11, 1368–1375 (1993).
Long, G. V. et al. Epacadostat (E) plus pembrolizumab (P) versus pembrolizumab alone in patients (pts) with unresectable or metastatic melanoma: results of the phase 3 ECHO-301/KEYNOTE-252 study. J. Clin. Oncol. 36, 108–108 (2018).
Yap, T. A. et al. Complex I inhibitor of oxidative phosphorylation in advanced solid tumors and acute myeloid leukemia: phase I trials. Nat. Med. 29, 115–126 (2023).
Lehuédé, C., Dupuy, F., Rabinovitch, R., Jones, R. G. & Siegel, P. M. Metabolic plasticity as a determinant of tumor growth and metastasis. Cancer Res. 76, 5201–5208 (2016).
Yan, H. et al. IDH1 and IDH2 mutations in gliomas. N. Engl. J. Med. 360, 765–773 (2009).
Stein, E. M. et al. Enasidenib in mutant IDH2 relapsed or refractory acute myeloid leukemia. Blood 130, 722–731 (2017).
Hsieh, J. J. et al. Renal cell carcinoma. Nat. Rev. Dis. Prim. 3, 17009 (2017).
Acknowledgements
M.C.S. is supported by P30CA016520, R35CA220483 and R01CA276512 from the NIH. N.J.C. is supported by F30CA271654 from the NIH.
Author information
Authors and Affiliations
Contributions
Both authors researched data for the article, made substantial contributions to discussions of the content and reviewed or edited the manuscript before submission. N.J.C. wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Nephrology thanks F. Baltazar, R. Deberardinis and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Glossary
- Heteroplasmic
-
A cell that has more than one mitochondrial genome.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Coffey, N.J., Simon, M.C. Metabolic alterations in hereditary and sporadic renal cell carcinoma. Nat Rev Nephrol 20, 233–250 (2024). https://doi.org/10.1038/s41581-023-00800-2
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41581-023-00800-2