Abstract
In rheumatoid arthritis (RA), the aging process of the immune system is accelerated. Formerly, this phenomenon was suspected to be a consequence of chronic inflammatory activity. However, newer data strongly suggest that deficiencies in maintaining telomeres and overall DNA stability cause excessive apoptosis of RA T cells, imposing proliferative pressure and premature aging on the system. Already during the early stages of their life cycle, and long before they participate in the inflammatory process, RA T cells are lost owing to increased apoptotic susceptibility. A search for underlying mechanisms has led to the discovery of defective pathways of repairing broken DNA and elongating and protecting telomeric sequences at the chromosomal ends. Two enzymatic machineries devoted to DNA repair and maintenance have been implicated. RA T cells fail to induce sufficient amounts of the telomeric repair enzyme telomerase, leaving telomeric ends uncapped and thus susceptible to damage. Of equal importance, RA T cells produce low levels of the DNA repair enzyme ataxia telangiectasia mutated and the complex of nucleoproteins that sense and fix DNA double-strand breaks. The inability to repair damaged DNA renders naive T cells vulnerable to apoptosis, exhausts T-cell regeneration and reshapes the T cell repertoire. Therapeutic attempts to reset the immune systems of patients with RA and prevent premature immunosenescence should include restoration of DNA repair capability.
Key Points
-
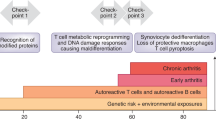
In rheumatoid arthritis (RA) naive T cells have shortened telomeres and contracted diversity, and memory T cells reach their differentiation potential, lose CD28 and acquire alternative regulator receptors
-
In RA, T cells' telomeric maintenance is dysfunctional, owing to insufficient production of the repair enzyme telomerase
-
RA T cells have a high load of DNA double-strand breaks, jeopardizing DNA integrity and cell survival
-
The failure to repair damaged DNA is caused by deficiency of the ataxia telangiectasia mutated (ATM)–MRE11–RAD50–NBS1 DNA repair machinery; mutations in ATM cause the immunodeficiency syndrome ataxia telangiectasia
-
The inability to maintain telomeric integrity and to repair broken DNA leads to excessive apoptotic death of RA T cells, and places the immune system under chronic proliferative stress
-
Recognizing that molecular deficiencies in nuclear stability can exhaust immune regeneration and lead to premature immune aging could change the pathogenetic concept of RA and advance the pursuit of novel therapies
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Cope, A. P. T cells in rheumatoid arthritis. Arthritis. Res. Ther. 10 (Suppl. 1), S1 (2008).
Brennan, F. M. & McInnes, I. B. Evidence that cytokines play a role in rheumatoid arthritis. J. Clin. Invest. 118, 3537–3545 (2008).
Isaacs, J. D. Therapeutic T-cell manipulation in rheumatoid arthritis: past, present and future. Rheumatology (Oxford) 47, 1461–1468 (2008).
Naz, S. M. & Symmons, D. P. Mortality in established rheumatoid arthritis. Best Pract. Res. Clin. Rheumatol. 21, 871–883 (2007).
Gabriel, S. E. Why do people with rheumatoid arthritis still die prematurely? Ann. Rheum. Dis. 67 (Suppl. 3), iii30–iii34 (2008).
Goronzy, J. J. & Weyand, C. M. Rheumatoid arthritis. Immunol. Rev. 204, 55–73 (2005).
Montecucco, F. & Mach, F. Common inflammatory mediators orchestrate pathophysiological processes in rheumatoid arthritis and atherosclerosis. Rheumatology (Oxford) 48, 11–22 (2009).
Warrington, K. J. et al. Rheumatoid arthritis is an independent risk factor for multi-vessel coronary artery disease: a case control study. Arthritis Res. Ther. 7, R984–R991 (2005).
Doran, M. F., Pond, G. R., Crowson, C. S., O'Fallon, W. M. & Gabriel, S. E. Trends in incidence and mortality in rheumatoid arthritis in Rochester, Minnesota, over a forty-year period. Arthritis Rheum. 46, 625–631 (2002).
Dorshkind, K., Montecino-Rodriguez, E. & Signer, R. A. The ageing immune system: is it ever too old to become young again? Nat. Rev. Immunol. 9, 57–62 (2009).
Larbi, A., Fulop, T. & Pawelec, G. Immune receptor signaling, aging and autoimmunity. Adv. Exp. Med. Biol. 640, 312–324 (2008).
Weyand, C. M. & Goronzy, J. J. Stem cell aging and autoimmunity in rheumatoid arthritis. Trends Mol. Med. 10, 426–433 (2004).
Minamino, T. & Komuro, I. Vascular aging: insights from studies on cellular senescence, stem cell aging, and progeroid syndromes. Nat. Clin. Pract. Cardiovasc. Med. 5, 637–648 (2008).
Flavell, S. J. et al. Fibroblasts as novel therapeutic targets in chronic inflammation. Br. J. Pharmacol. 153 (Suppl. 1), S241–S466 (2008).
Miossec, P. Dynamic interactions between T cells and dendritic cells and their derived cytokines/chemokines in the rheumatoid synovium. Arthritis Res. Ther. 10 (Suppl.) 1, S2 (2008).
Yanaba, K. et al. B-lymphocyte contributions to human autoimmune disease. Immunol. Rev. 223, 284–299 (2008).
Weyand, C. M., Fulbright, J. W. & Goronzy, J. J. Immunosenescence, autoimmunity, and rheumatoid arthritis. Exp. Gerontol. 38, 833–841 (2003).
Fujii, H., Shao, L., Colmegna, I., Goronzy, J. J. & Weyand, C. M. Telomerase insufficiency in rheumatoid arthritis. Proc. Natl Acad. Sci. USA 106, 4360–4365 (2009).
Shao, L. et al. Deficiency of the DNA repair enzyme ATM in rheumatoid arthritis. J. Exp. Med. 206, 1435–1449 (2009).
Jendro, M. C., Ganten, T., Matteson, E. L., Weyand, C. M. & Goronzy, J. J. Emergence of oligoclonal T cell populations following therapeutic T cell depletion in rheumatoid arthritis. Arthritis Rheum. 38, 1242–1251 (1995).
Brett, S. et al. Repopulation of blood lymphocyte sub-populations in rheumatoid arthritis patients treated with the depleting humanized monoclonal antibody, CAMPATH-1H. Immunology 88, 13–19 (1996).
Lorenzi, A. R. et al. Morbidity and mortality in rheumatoid arthritis patients with prolonged therapy-induced lymphopenia: twelve-year outcomes. Arthritis Rheum. 58, 370–375 (2008).
Naylor, K. et al. The influence of age on T cell generation and TCR diversity. J. Immunol. 174, 7446–7452 (2005).
Schonland, S. O. et al. Homeostatic control of T-cell generation in neonates. Blood 102, 1428–1434 (2003).
Wagner, U. G., Koetz, K., Weyand, C. M. & Goronzy, J. J. Perturbation of the T cell repertoire in rheumatoid arthritis. Proc. Natl Acad. Sci. USA 95, 14447–14452 (1998).
Koetz, K. et al. T cell homeostasis in patients with rheumatoid arthritis. Proc. Natl Acad. Sci. USA 97, 9203–9208 (2000).
Ponchel, F. et al. Dysregulated lymphocyte proliferation and differentiation in patients with rheumatoid arthritis. Blood 100, 4550–4556 (2002).
Weyand, C. M. & Goronzy, J. J. T-cell-targeted therapies in rheumatoid arthritis. Nat. Clin. Pract. Rheumatol. 2, 201–210 (2006).
Schmidt, D., Goronzy, J. J. & Weyand, C. M. CD4+CD7−CD28− T cells are expanded in rheumatoid arthritis and are characterized by autoreactivity. J. Clin. Invest. 97, 2027–2037 (1996).
Liuzzo, G. et al. Monoclonal T-cell proliferation and plaque instability in acute coronary syndromes. Circulation 101, 2883–2888 (2000).
Steer, S. E. et al. Reduced telomere length in rheumatoid arthritis is independent of disease activity and duration. Ann. Rheum. Dis. 66, 476–480 (2007).
Salmon, M. & Akbar, A. N. Telomere erosion: a new link between HLA DR4 and rheumatoid arthritis? Trends Immunol. 25, 339–341 (2004).
Palm, W. & de Lange, T. How shelterin protects mammalian telomeres. Annu. Rev. Genet. 42, 301–334 (2008).
Sabourin, M. et al. Yeast recombination pathways triggered by topoisomerase II-mediated DNA breaks. Nucleic Acids Res. 31, 4373–4384 (2003).
Schonland, S. O. et al. Premature telomeric loss in rheumatoid arthritis is genetically determined and involves both myeloid and lymphoid cell lineages. Proc. Natl Acad. Sci. USA 100, 13471–13476 (2003).
Colmegna, I. et al. Defective proliferative capacity and accelerated telomeric loss of hematopoietic progenitor cells in rheumatoid arthritis. Arthritis Rheum. 58, 990–1000 (2008).
Park, J. I. et al. Telomerase modulates Wnt signalling by association with target gene chromatin. Nature 460, 66–72 (2009).
Cohn, M. A. & D'Andrea, A. D. Chromatin recruitment of DNA repair proteins: lessons from the fanconi anemia and double-strand break repair pathways. Mol. Cell 32, 306–312 (2008).
Kitagawa, R. & Kastan, M. B. The ATM-dependent DNA damage signaling pathway. Cold Spring Harb. Symp. Quant. Biol. 70, 99–109 (2005).
Lavin, M. F. ATM and the Mre11 complex combine to recognize and signal DNA double-strand breaks. Oncogene 26, 7749–7758 (2007).
Cimprich, K. A. & Cortez, D. ATR: an essential regulator of genome integrity. Nat. Rev. Mol. Cell Biol. 9, 616–627 (2008).
Andrews, N. P., Goronzy, J. J. & Weyand, C. M. Telomeres in immunological diseases of aging. Gerontology (in press).
Siddiqa, A., Cavazos, D. A. & Marciniak, R. A. Targeting telomerase. Rejuvenation Res. 9, 378–390 (2006).
Hahn, W. C. & Meyerson, M. Telomerase activation, cellular immortalization and cancer. Ann. Med. 33, 123–129 (2001).
Lo, H. W., Day, C. P. & Hung, M. C. Cancer-specific gene therapy. Adv. Genet. 54, 235–255 (2005).
Lavin, M. F. Ataxia-telangiectasia: from a rare disorder to a paradigm for cell signalling and cancer. Nat. Rev. Mol. Cell Biol. 9, 759–769 (2008).
Lavin, M. F. et al. ATM and cellular response to DNA damage. Adv. Exp. Med. Biol. 570, 457–476 (2005).
Lavin, M. F. & Shiloh, Y. The genetic defect in ataxia-telangiectasia. Annu. Rev. Immunol. 15, 177–202 (1997).
Maclean, K. H., Kastan, M. B. & Cleveland, J. L. ATM deficiency affects both apoptosis and proliferation to augment Myc-induced lymphomagenesis. Mol. Cancer Res. 5, 705–711 (2007).
Acknowledgements
The authors wish to thank Linda Arneson and Tamela Yeargin for their editorial support. This work was funded in part by grants from the NIH (AR 42527, AR 41974, AI 44142, AI 57266, AG 15043) and the “Within Our Reach” campaign of the American College of Rheumatology Research and Education Foundation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Weyand, C., Fujii, H., Shao, L. et al. Rejuvenating the immune system in rheumatoid arthritis. Nat Rev Rheumatol 5, 583–588 (2009). https://doi.org/10.1038/nrrheum.2009.180
Issue Date:
DOI: https://doi.org/10.1038/nrrheum.2009.180
This article is cited by
-
Small heterodimer partner interacting leucine zipper protein (SMILE) ameliorates autoimmune arthritis via AMPK signaling pathway and the regulation of B cell activation
Cell Communication and Signaling (2023)
-
The immunology of rheumatoid arthritis
Nature Immunology (2021)
-
Frailty as a novel predictor of achieving comprehensive disease control (CDC) in rheumatoid arthritis
Clinical Rheumatology (2021)
-
Protective effect of Corynoline on the CFA induced Rheumatoid arthritis via attenuation of oxidative and inflammatory mediators
Molecular and Cellular Biochemistry (2021)
-
Immunology of the ageing kidney
Nature Reviews Nephrology (2019)