Abstract
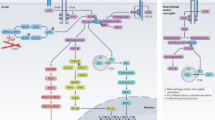
Multiple sclerosis (MS) is a debilitating neurological disorder involving autoimmune destruction of myelin. Although the pathogenic mechanisms underlying MS are not fully understood, T cells are thought to have a key role in orchestrating the aberrant CNS-directed adaptive immune response in the early and relapsing–remitting phase of disease. New therapeutic interventions with improved efficacy over existing drugs and good tolerability are needed. A promising therapy under investigation is daclizumab—a humanized monoclonal antibody directed against the IL-2 receptor α chain (CD25). Clinical trials have shown that daclizumab strongly inhibits disease activity and slows disease progression in MS. Novel and intriguing mechanisms of action of daclizumab have been identified that might explain its clinical efficacy—namely, expansion and enhancement of the immune regulatory function of CD56bright natural killer cells, reduction of early T-cell activation through blockade of IL-2 cross-presentation by dendritic cells, and reduction of lymphoid tissue inducer cells—thereby enhancing endogenous mechanisms of immune tolerance. This Review discusses the efficacy and safety of daclizumab in patients with MS and provides a detailed insight into the multifunctional mechanisms of action of this drug.
Key Points
-
Daclizumab is an immune-modulating humanized IgG1 monoclonal antibody directed against the IL-2 receptor α chain (IL-2Rα, also known as CD25) that is under investigation for treatment of multiple sclerosis (MS)
-
Binding of daclizumab to IL-2Rα prevents assembly and signalling of the high-affinity IL-2R, which is involved in immune system activation
-
In clinical trials, daclizumab strongly inhibited disease activity and slowed disease progression in patients with MS, with a fairly good tolerability and safety profile
-
Novel mechanisms of action whereby daclizumab modulates the immune system in MS have been identified
-
These mechanisms involve expansion and stimulation of immune regulatory CD56bright natural killer cells, reduction of early T-cell activation, and reduction in the number of proinflammatory lymphoid tissue inducer cells
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Compston, A. & Coles, A. Multiple sclerosis. Lancet 359, 1221–1231 (2002).
Frohman, E. M., Racke, M. K. & Raine, C. S. Multiple sclerosis—the plaque and its pathogenesis. N. Engl. J. Med. 354, 942–955 (2006).
Goverman, J. M. Immune tolerance in multiple sclerosis. Immunol. Rev. 241, 228–240 (2011).
Miller, E. Multiple sclerosis. Adv. Exp. Med. Biol. 724, 222–238 (2012).
Zozulya, A. L. & Wiendl, H. The role of regulatory T cells in multiple sclerosis. Nat. Clin. Pract. Neurol. 4, 384–398 (2008).
Cheng, G., Yu, A. & Malek, T. R. T-cell tolerance and the multi-functional role of IL-2R signaling in T-regulatory cells. Immunol. Rev. 241, 63–76 (2011).
Malek, T. R. & Castro, I. Interleukin-2 receptor signaling: at the interface between tolerance and immunity. Immunity 33, 153–165 (2010).
Matesanz, F. et al. Allelic expression and interleukin-2 polymorphisms in multiple sclerosis. J. Neuroimmunol. 119, 101–105 (2001).
Reboul, J. et al. Cytokines in genetic susceptibility to multiple sclerosis: a candidate gene approach. French Multiple Sclerosis Genetics Group. J. Neuroimmunol. 102, 107–112 (2000).
Malek, T. R. The biology of interleukin-2. Annu. Rev. Immunol. 26, 453–479 (2008).
Waldmann, T. A. Anti-Tac (daclizumab, Zenapax) in the treatment of leukemia, autoimmune diseases, and in the prevention of allograft rejection: a 25-year personal odyssey. J. Clin. Immunol. 27, 1–18 (2007).
Waldmann, T. A. The IL-2/IL-15 receptor systems: targets for immunotherapy. J. Clin. Immunol. 22, 51–56 (2002).
Nussenblatt, R. B. et al. Treatment of noninfectious intermediate and posterior uveitis with the humanized anti-Tac mAb: a phase I/II clinical trial. Proc. Natl Acad. Sci. USA 96, 7462–7466 (1999).
Nussenblatt, R. B. et al. Humanized anti-interleukin-2 (IL-2) receptor alpha therapy: long-term results in uveitis patients and preliminary safety and activity data for establishing parameters for subcutaneous administration. J. Autoimmun. 21, 283–293 (2003).
Bielekova, B. et al. Effect of anti-CD25 antibody daclizumab in the inhibition of inflammation and stabilization of disease progression in multiple sclerosis. Arch. Neurol. 66, 483–489 (2009).
Bielekova, B. et al. Intrathecal effects of daclizumab treatment of multiple sclerosis. Neurology 77, 1877–1886 (2011).
Bielekova, B. et al. Humanized anti-CD25 (daclizumab) inhibits disease activity in multiple sclerosis patients failing to respond to interferon β. Proc. Natl Acad. Sci. USA 101, 8705–8708 (2004).
Rojas, M. A., Carlson, N. G., Miller, T. L. & Rose, J. W. Long-term daclizumab therapy in relapsing–remitting multiple sclerosis. Ther. Adv. Neurol. Disord. 2, 291–297 (2009).
Rose, J. W. et al. Daclizumab phase II trial in relapsing and remitting multiple sclerosis: MRI and clinical results. Neurology 69, 785–789 (2007).
Rose, J. W., Watt, H. E., White, A. T. & Carlson, N. G. Treatment of multiple sclerosis with an anti-interleukin-2 receptor monoclonal antibody. Ann. Neurol. 56, 864–867 (2004).
Wynn, D. et al. Daclizumab in active relapsing multiple sclerosis (CHOICE study): a phase 2, randomised, double-blind, placebo-controlled, add-on trial with interferon beta. Lancet Neurol. 9, 381–390 (2010).
Gold, R. et al. Daclizumab high-yield process in relapsing-remitting multiple sclerosis (SELECT): a randomised, double-blind placebo-cotrolled trial. Lancet http://dx.doi.org/10.1016/S0140-6736(12)62190-4.
Giovannoni, G. et al. Primary results of the SELECTION trial of daclizumab HYP in relapsing multiple sclerosis. Mult. Scler. 18, 514 (2012).
Oh, U. et al. Regulatory T cells are reduced during anti-CD25 antibody treatment of multiple sclerosis. Arch. Neurol. 66, 471–479 (2009).
Bielekova, B. et al. Regulatory CD56bright natural killer cells mediate immunomodulatory effects of IL-2Rα-targeted therapy (daclizumab) in multiple sclerosis. Proc. Natl Acad. Sci. USA 103, 5941–5946 (2006).
Martin, J. F., Perry, J. S., Jakhete, N. R., Wang, X. & Bielekova, B. An IL-2 paradox: blocking CD25 on T cells induces IL-2-driven activation of CD56bright NK cells. J. Immunol. 185, 1311–1320 (2010).
Perry, J. S. et al. Inhibition of LTi cell development by CD25 blockade is associated with decreased intrathecal inflammation in multiple sclerosis. Sci. Transl. Med. 4, 145ra106 (2012).
Sheridan, J. P. et al. Intermediate-affinity interleukin-2 receptor expression predicts CD56bright natural killer cell expansion after daclizumab treatment in the CHOICE study of patients with multiple sclerosis. Mult. Scler. 17, 1441–1448 (2011).
Wuest, S. C. et al. A role for interleukin-2 trans-presentation in dendritic cell-mediated T cell activation in humans, as revealed by daclizumab therapy. Nat. Med. 17, 604–609 (2011).
Martin, R. Humanized anti-CD25 antibody treatment with daclizumab in multiple sclerosis. Neurodegener. Dis. 5, 23–26 (2008).
Martin, R. Anti-CD25 (daclizumab) monoclonal antibody therapy in relapsing-remitting multiple sclerosis. Clin. Immunol. 142, 9–14 (2012).
Sadlack, B. et al. Ulcerative colitis-like disease in mice with a disrupted interleukin-2 gene. Cell 75, 253–261 (1993).
Sadlack, B. et al. Generalized autoimmune disease in interleukin-2-deficient mice is triggered by an uncontrolled activation and proliferation of CD4+ T cells. Eur. J. Immunol. 25, 3053–3059 (1995).
Suzuki, H. et al. Deregulated T cell activation and autoimmunity in mice lacking interleukin-2 receptor β. Science 268, 1472–1476 (1995).
Malek, T. R., Yu, A., Vincek, V., Scibelli, P. & Kong, L. CD4 regulatory T cells prevent lethal autoimmunity in IL-2Rβ-deficient mice. Implications for the nonredundant function of IL-2. Immunity 17, 167–178 (2002).
Bayer, A. L., Yu, A. & Malek, T. R. Function of the IL-2R for thymic and peripheral CD4+CD25+ Foxp3+ T regulatory cells. J. Immunol. 178, 4062–4071 (2007).
Hafler, D. A. et al. Risk alleles for multiple sclerosis identified by a genomewide study. N. Engl. J. Med. 357, 851–862 (2007).
Lowe, C. E. et al. Large-scale genetic fine mapping and genotype–phenotype associations implicate polymorphism in the IL2RA region in type 1 diabetes. Nat. Genet. 39, 1074–1082 (2007).
Maier, L. M. et al. IL2RA genetic heterogeneity in multiple sclerosis and type 1 diabetes susceptibility and soluble interleukin-2 receptor production. PLoS Genet. 5, e1000322 (2009).
Roifman, C. M. Human IL-2 receptor α chain deficiency. Pediatr. Res. 48, 6–11 (2000).
Sharfe, N., Dadi, H. K., Shahar, M. & Roifman, C. M. Human immune disorder arising from mutation of the α chain of the interleukin-2 receptor. Proc. Natl Acad. Sci. USA 94, 3168–3171 (1997).
Caudy, A. A., Reddy, S. T., Chatila, T., Atkinson, J. P. & Verbsky, J. W. CD25 deficiency causes an immune dysregulation, polyendocrinopathy, enteropathy, X-linked-like syndrome, and defective IL-10 expression from CD4 lymphocytes. J. Allergy Clin. Immunol. 119, 482–487 (2007).
Fontenot, J. D., Rasmussen, J. P., Gavin, M. A. & Rudensky, A. Y. A function for interleukin 2 in Foxp3-expressing regulatory T cells. Nat. Immunol. 6, 1142–1151 (2005).
Williams, M. A., Tyznik, A. J. & Bevan, M. J. Interleukin-2 signals during priming are required for secondary expansion of CD8+ memory T cells. Nature 441, 890–893 (2006).
Stauber, D. J., Debler, E. W., Horton, P. A., Smith, K. A. & Wilson, I. A. Crystal structure of the IL-2 signaling complex: paradigm for a heterotrimeric cytokine receptor. Proc. Natl Acad. Sci. USA 103, 2788–2793 (2006).
Hemar, A. et al. Endocytosis of interleukin 2 receptors in human T lymphocytes: distinct intracellular localization and fate of the receptor α, β, and γ chains. J. Cell Biol. 129, 55–64 (1995).
Yu, A. & Malek, T. R. The proteasome regulates receptor-mediated endocytosis of interleukin-2. J. Biol. Chem. 276, 381–385 (2001).
Gorman, M. P., Tillema, J. M., Ciliax, A. M., Guttmann, C. R. & Chitnis, T. Daclizumab use in patients with pediatric multiple sclerosis. Arch. Neurol. 69, 78–81 (2012).
Bielekova, B. Daclizumab therapy for multiple sclerosis. Neurotherapeutics 10, 55–67 (2013).
Giovannoni, G. et al. Increase in proportion of patients free from disease activity following 1 year treatment with daclizumab high-yield process in relapsing-remitting multiple sclerosis: results from the SELECT study. Mult. Scler. 18 (Suppl. 4), 419 (2012).
Carbone, T. et al. CD56highCD16−CD62L− NK cells accumulate in allergic contact dermatitis and contribute to the expression of allergic responses. J. Immunol. 184, 1102–1110 (2010).
US National Library of Medicine. Efficacy and safety of daclizumab high yield process versus interferon β 1a in patients with relapsing–remitting multiple sclerosis (DECIDE). ClinicalTrials.gov [online], (2012).
Farag, S. S. & Caligiuri, M. A. Human natural killer cell development and biology. Blood Rev. 20, 123–137 (2006).
Cooper, M. A., Fehniger, T. A. & Caligiuri, M. A. The biology of human natural killer-cell subsets. Trends Immunol. 22, 633–640 (2001).
Cooper, M. A. et al. Human natural killer cells: a unique innate immunoregulatory role for the CD56bright subset. Blood 97, 3146–3151 (2001).
Fehniger, T. A. et al. CD56bright natural killer cells are present in human lymph nodes and are activated by T cell-derived IL-2: a potential new link between adaptive and innate immunity. Blood 101, 3052–3057 (2003).
Airas, L. et al. Immunoregulatory factors in multiple sclerosis patients during and after pregnancy: relevance of natural killer cells. Clin. Exp. Immunol. 151, 235–243 (2008).
Della Chiesa, M. et al. The natural killer cell-mediated killing of autologous dendritic cells is confined to a cell subset expressing CD94/NKG2A, but lacking inhibitory killer Ig-like receptors. Eur. J. Immunol. 33, 1657–1666 (2003).
Lünemann, A. et al. Human NK cells kill resting but not activated microglia via NKG2D- and NKp46-mediated recognition. J. Immunol. 181, 6170–6177 (2008).
Hao, J. et al. Central nervous system (CNS)-resident natural killer cells suppress Th17 responses and CNS autoimmune pathology. J. Exp. Med. 207, 1907–1921 (2010).
Fletcher, J. M., Lalor, S. J., Sweeney, C. M., Tubridy, N. & Mills, K. H. T cells in multiple sclerosis and experimental autoimmune encephalomyelitis. Clin. Exp. Immunol. 162, 1–11 (2010).
Steinman, L. Assessment of animal models for MS and demyelinating disease in the design of rational therapy. Neuron 24, 511–514 (1999).
Hao, J. et al. Interleukin-2/interleukin-2 antibody therapy induces target organ natural killer cells that inhibit central nervous system inflammation. Ann. Neurol. 69, 721–734 (2011).
Vivier, E., Tomasello, E., Baratin, M., Walzer, T. & Ugolini, S. Functions of natural killer cells. Nat. Immunol. 9, 503–510 (2008).
Hamann, I. et al. Characterization of natural killer cells in paired CSF and blood samples during neuroinflammation. J. Neuroimmunol. 254, 165–169 (2013).
Elkins, J., Sheridan, J., Amaravadi, L., Riester, K. & O'Neill, G. CD56bright natural killer cell expansion predicts response to daclizumab HYP treatment in RRMS: results of the SELECT trial. Neurology 78 (Meeting Abstracts 1), S31.004 (2012).
Jiang, W., Chai, N. R., Maric, D. & Bielekova, B. Unexpected role for granzyme K in CD56bright NK cell-mediated immunoregulation of multiple sclerosis. J. Immunol. 187, 781–790 (2011).
Bratke, K., Kuepper, M., Bade, B., Virchow, J. C. Jr & Luttmann, W. Differential expression of human granzymes A, B, and K in natural killer cells and during CD8+ T cell differentiation in peripheral blood. Eur. J. Immunol. 35, 2608–2616 (2005).
Zhao, T. et al. Granzyme K cleaves the nucleosome assembly protein SET to induce single-stranded DNA nicks of target cells. Cell Death Differ. 14, 489–499 (2007).
Bovenschen, N. et al. Granzyme K displays highly restricted substrate specificity that only partially overlaps with granzyme A. J. Biol. Chem. 284, 3504–3512 (2009).
De Jager, P. L. et al. Cytometric profiling in multiple sclerosis uncovers patient population structure and a reduction of CD8low cells. Brain 131, 1701–1711 (2008).
Lünemann, A. et al. Impaired IFN-γ production and proliferation of NK cells in multiple sclerosis. Int. Immunol. 23, 139–148 (2011).
Benczur, M. et al. Dysfunction of natural killer cells in multiple sclerosis: a possible pathogenetic factor. Clin. Exp. Immunol. 39, 657–662 (1980).
Cupedo, T. et al. Human fetal lymphoid tissue-inducer cells are interleukin 17-producing precursors to RORC+ CD127+ natural killer-like cells. Nat. Immunol. 10, 66–74 (2009).
Lucchinetti, C. F. et al. Inflammatory cortical demyelination in early multiple sclerosis. N. Engl. J. Med. 365, 2188–2197 (2011).
Spits, H. & Di Santo, J. P. The expanding family of innate lymphoid cells: regulators and effectors of immunity and tissue remodeling. Nat. Immunol. 12, 21–27 (2011).
Sawa, S. et al. Lineage relationship analysis of RORγt+ innate lymphoid cells. Science 330, 665–669 (2010).
Spits, H. & Cupedo, T. Innate lymphoid cells: emerging insights in development, lineage relationships, and function. Annu. Rev. Immunol. 30, 647–675 (2012).
Freud, A. G. et al. A human CD34+ subset resides in lymph nodes and differentiates into CD56bright natural killer cells. Immunity 22, 295–304 (2005).
Rose, J. W. Anti-CD25 immunotherapy: regulating the regulators. Sci. Transl. Med. 4, 145fs25 (2012).
Schluns, K. S. Window of opportunity for daclizumab. Nat. Med. 17, 545–547 (2011).
Mnasria, K. et al. Anti-CD25 antibodies affect cytokine synthesis pattern of human dendritic cells and decrease their ability to prime allogeneic CD4+ T cells. J. Leukoc. Biol. 84, 460–467 (2008).
Rech, A. J. & Vonderheide, R. H. Clinical use of anti-CD25 antibody daclizumab to enhance immune responses to tumor antigen vaccination by targeting regulatory T cells. Ann. N. Y. Acad. Sci. 1174, 99–106 (2009).
Gold, R. et al. Regulatory T-cell numbers in patients with RRMS were not associated with clinical or MRI outcomes in the SELECT study. Mult. Scler. 18 (Suppl. 4), 195 (2012).
Mehta, D. et al. Reversal of the pharmacodynamic effects of daclizumab HYP following treatment washout: results from the SLECTION study. Neurology 80 (Meeting Abstracts 1), P05.188 (2013).
Yu, A., Zhu, L., Altman, N. H. & Malek, T. R. A low interleukin-2 receptor signaling threshold supports the development and homeostasis of T regulatory cells. Immunity 30, 204–217 (2009).
Bayer, A. L., Lee, J. Y., de la Barrera, A., Surh, C. D. & Malek, T. R. A function for IL-7R for CD4+CD25+Foxp3+ T regulatory cells. J. Immunol. 181, 225–234 (2008).
Heninger, A. K. et al. IL-7 abrogates suppressive activity of human CD4+CD25+FOXP3+ regulatory T cells and allows expansion of alloreactive and autoreactive T cells. J. Immunol. 189, 5649–5658 (2012).
Sakaguchi, S., Miyara, M., Costantino, C. M. & Hafler, D. A. FOXP3+ regulatory T cells in the human immune system. Nat. Rev. Immunol. 10, 490–500 (2010).
Rech, A. J. et al. CD25 blockade depletes and selectively reprograms regulatory T cells in concert with immunotherapy in cancer patients. Sci. Transl. Med. 4, 134ra62 (2012).
Leonard, W. J. et al. A monoclonal antibody that appears to recognize the receptor for human T-cell growth factor; partial characterization of the receptor. Nature 300, 267–269 (1982).
Depper, J. M., Leonard, W. J., Robb, R. J., Waldmann, T. A. & Greene, W. C. Blockade of the interleukin-2 receptor by anti-Tac antibody: inhibition of human lymphocyte activation. J. Immunol. 131, 690–696 (1983).
McDyer, J. F. et al. IL-2 receptor blockade inhibits late, but not early, IFN-γ and CD40 ligand expression in human T cells: disruption of both IL-12-dependent and -independent pathways of IFN-γ production. J. Immunol. 169, 2736–2746 (2002).
Tkaczuk, J. et al. Intracellular signaling consequences of anti-IL-2Rα blockade by daclizumab. Transplant. Proc. 33, 212–213 (2001).
Rani, A. et al. IL-2 regulates expression of C-MAF in human CD4 T cells. J. Immunol. 187, 3721–3729 (2011).
Sheridan, J. P. et al. Daclizumab treatment reduces activated T cells: results from the CHOICE MS study. Neurology 72, A35–A35 (2009).
Wiendl, H. & Kieseier, B. Multiple sclerosis: Reprogramming the immune repertoire with alemtuzumab in MS. Nat. Rev. Neurol. 9, 125–126 (2013).
Liu, J., Wang, L., Zhan, S.-Y. & Xia, Y. Daclizumab for relapsing remitting multiple sclerosis. Cochrane Database of Systematic Reviews, Issue 4. Art. No.: CD008127. http://dx.doi.org/10.1002/14651858.CD008127.pub3.
Acknowledgements
H. Wiendl is supported by the German Research Foundation (individual research grant “The role of NK cells in the immune regulation of MS” and centre grants CRC128 “Multiple sclerosis” and SFB1009 “Breaking barriers”), the German Competence Network of MS (KKNMS) founded by the German Federal Ministry of Education and Research (BMBF), the European Union (BEST-MS), the Interdisciplinary Clinical Research Centre Münster (IZKF) and the Interdisciplinary Centre for Clinical Research Münster (IMF). C. C. Gross is supported by the German Research Foundation (individual research grant “The role of NK cells in the immune regulation of MS”). We thank A. Fitton and P. Lane of UBC Scientific Solutions, a company that has received support from Biogen Idec, for assistance with searching of the literature, drafting of the figures, and editing of the manuscript for language prior to submission.
Author information
Authors and Affiliations
Contributions
Both authors researched the data for the article, provided a substantial contribution to discussions of the content, and contributed to writing and to review and/or editing of the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
H. Wiendl has received honoraria for lecturing, and travel expenses for attending meetings from Bayer Health Care, Biogen Idec/Elan Corporation, Lilly, Lundbeck, Merck Serono, Novartis, Sanofi Aventis, and TEVA Neuroscience; has served or serves as a consultant for Biogen Idec, Merck Serono, Novartis Pharma, Sanofi-Aventis; and receives research support from Bayer Schering Pharma, Biogen Idec/Elan Corporation, Merck Serono, Novartis, Novo Nordisk, Sanofi-Aventis. C. C. Gross has received travel expenses for attending meetings from Novartis Pharma.
Rights and permissions
About this article
Cite this article
Wiendl, H., Gross, C. Modulation of IL-2Rα with daclizumab for treatment of multiple sclerosis. Nat Rev Neurol 9, 394–404 (2013). https://doi.org/10.1038/nrneurol.2013.95
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrneurol.2013.95
This article is cited by
-
Impact of disease-modifying therapy on dendritic cells and exploring their immunotherapeutic potential in multiple sclerosis
Journal of Neuroinflammation (2022)
-
What Have Failed, Interrupted, and Withdrawn Antibody Therapies in Multiple Sclerosis Taught Us?
Neurotherapeutics (2022)
-
Established and Emerging Immunological Complications of Biological Therapeutics in Multiple Sclerosis
Drug Safety (2019)
-
Daclizumab: Development, Clinical Trials, and Practical Aspects of Use in Multiple Sclerosis
Neurotherapeutics (2017)
-
Combination Immunotherapy for Type 1 Diabetes
Current Diabetes Reports (2017)