Abstract
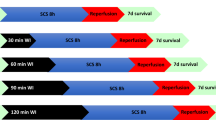
Optimizing kidney preservation is a primary issue in transplantation, particularly in relation to new donor sources, such as expanded criteria donors (ECDs) and donation after cardiac death (DCD). Kidneys from these donors are highly sensitive to ischemia–reperfusion injuries—the emblematic lesions encountered during transplantation. Despite years of research, static cold storage, with solutions designed in the 1980s, remains the gold standard in kidney transplantation. This kind of preservation, however, is unable to fully protect an ECD or DCD kidney, highlighting the need for novel strategies to improve kidney preservation or promote kidney recovery. This Review provides an overview of the emerging strategies to prevent ischemia–reperfusion injuries in donor kidneys and describes strategies that are aimed at the donor, organ or recipient to improve graft outcome. These approaches include management of donors, preconditioning of the kidney, improvements in organ preservation solutions, postconditioning and regenerative therapies of the kidney graft following transplantation. In addition, machine perfusion provides an interesting opportunity to evaluate kidney graft quality before transplantation. Overall, a combination of therapeutic approaches seem to provide the best outcome, but preclinical studies using relevant models are needed before these approaches can be incorporated into clinical practice.
Key Points
-
Donor resuscitation affects kidney function before and after transplantation
-
Remote ischemic preconditioning to reduce ischemia–reperfusion injuries in donor kidneys needs to be evaluated in animal models of transplantation
-
Cold storage solutions based on polyethylene glycols or machine perfusion should be used to preserve grafts from all types of donors
-
Overexpression of proteins involved in downregulating inflammation, oxidative stress and apoptosis using ex vivo gene therapy improves graft survival or function in animal models of transplantation
-
Multivariate analysis of perfusates using proton NMR spectroscopy could reveal a factor that is predictive of graft outcome
-
Kidney transplantation outcome could be improved by remote ischemic postconditioning, pharmacological treatments or small interfering RNA therapy; stem cell therapy might promote kidney repair in the recipient
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Salahudeen, A. K. Cold ischemic injury of transplanted kidneys: new insights from experimental studies. Am. J. Physiol. Renal Physiol. 287, F181–F187 (2004).
Salahudeen, A. K. Cold ischemic injury of transplanted organs: some new strategies against an old problem. Am. J. Transplant. 4, 1 (2004).
Perico, N., Cattaneo, D., Sayegh, M. H. & Remuzzi, G. Delayed graft function in kidney transplantation. Lancet 364, 1814–1827 (2004).
Smith, M. & Vyas, H. Management of the potential organ donor. Paediatr. Child Health 21, 182–186 (2011).
Westendorp, W. H., Leuvenink, H. G. & Ploeg, R. J. Brain death induced renal injury. Curr. Opin. Organ Transplant. 16, 151–156 (2011).
Gasser, M., Waaga, A. M., Laskowski, A. A. & Tilney, N. L. Organ transplantation from brain-dead donors: its impact on short- and long-term outcome revisited. Transplant. Rev. 15, 1–10 (2001).
Pratschke, J. et al. Influence of donor brain death on chronic rejection of renal transplants in rats. J. Am. Soc. Nephrol. 12, 2474–2481 (2001).
Giral, M. et al. Effect of brain-dead donor resuscitation on delayed graft function: results of a monocentric analysis. Transplantation 83, 1174–1181 (2007).
Ranasinghe, A. M. & Bonser, R. S. Endocrine changes in brain death and transplantation. Best Pract. Res. Clin. Endocrinol. Metab. 25, 799–812 (2011).
Bouma, H. R., Ploeg, R. J. & Schuurs, T. A. Signal transduction pathways involved in brain death-induced renal injury. Am. J. Transplant. 9, 989–997 (2009).
Blasco, V. et al. Comparison of the novel hydroxyethylstarch 130/0.4 and hydroxyethylstarch 200/0.6 in brain-dead donor resuscitation on renal function after transplantation. Br. J. Anaesth. 100, 504–508 (2008).
Blasco, V. et al. Impact of intensive care on renal function before graft harvest: results of a monocentric study. Crit. Care 11, R103 (2007).
Damman, J. et al. Targeting complement activation in brain-dead donors improves renal function after transplantation. Transpl. Immunol. 24, 233–237 (2011).
Pratschke, J. et al. Improvements in early behavior of rat kidney allografts after treatment of the brain-dead donor. Ann. Surg. 234, 732–740 (2001).
Preissler, G. et al. Targeted endothelial delivery of nanosized catalase immunoconjugates protects lung grafts donated after cardiac death. Transplantation 92, 380–387 (2011).
Chatauret, N., Thuillier, R. & Hauet, T. Preservation strategies to reduce ischemic injury in kidney transplantation: pharmacological and genetic approaches. Curr. Opin. Organ Transplant. 16, 180–187 (2011).
Valero, R. et al. Blood flow and oxygen extraction during normothermic recirculation and total body cooling predict viability of liver from non-heart-beating pig donors. Transplant. Proc. 29, 3469–3470 (1997).
Valero, R. et al. Normothermic recirculation reduces primary graft dysfunction of kidneys obtained from non-heart-beating donors. Transpl. Int. 13, 303–310 (2000).
Magliocca, J. F. et al. Extracorporeal support for organ donation after cardiac death effectively expands the donor pool. J. Trauma 58, 1095–1101 (2005).
Imber, C. J. et al. Advantages of normothermic perfusion over cold storage in liver preservation. Transplantation 73, 701–709 (2002).
Hosgood, S. A. & Nicholson, M. L. Normothermic kidney preservation. Curr. Opin. Organ Transplant. 16, 169–173 (2011).
Huang, Y. et al. Can ischemic preconditioning alone really protect organs from ischemia reperfusion injury in transplantation. Transpl. Immunol. 20, 127–131 (2009).
Hausenloy, D. J. & Yellon, D. M. The therapeutic potential of ischemic conditioning: an update. Nat. Rev. Cardiol. 8, 619–629 (2011).
Zimmerman, R. F. et al. Ischemic preconditioning at a remote site prevents acute kidney injury in patients following cardiac surgery. Kidney Int. 80, 861–867 (2011).
Walsh, S. R. et al. Remote ischemic preconditioning for renal and cardiac protection during endovascular aneurysm repair: a randomized controlled trial. J. Endovasc. Ther. 16, 680–689 (2009).
Li, L. et al. Remote perconditioning reduces myocardial injury in adult valve replacement: a randomized controlled trial. J. Surg. Res. 164, e21–e26 (2010).
Rahman, I. A. et al. Remote ischemic preconditioning in human coronary artery bypass surgery: from promise to disappointment? Circulation 122 (Suppl.), S53–S59 (2010).
Choi, Y. S. et al. Effect of remote ischemic preconditioning on renal dysfunction after complex valvular heart surgery: a randomized controlled trial. J. Thorac. Cardiovasc. Surg. 142, 148–154 (2011).
Walsh, S. R. et al. Remote ischemic preconditioning for renal protection during elective open infrarenal abdominal aortic aneurysm repair: randomized controlled trial. Vasc. Endovasc. Surg. 44, 334–340 (2010).
Wever, K. E. et al. Remote ischaemic preconditioning by brief hind limb ischaemia protects against renal ischaemia-reperfusion injury: the role of adenosine. Nephrol. Dial. Transplant. 26, 3108–3117 (2011).
Anaya-Prado, R. & Delgado-Vazquez, J. A. Scientific basis of organ preservation. Curr. Opin. Organ Transplant. 13, 129–134 (2008).
Jamieson, R. W. & Friend, P. J. Organ reperfusion and preservation. Front. Biosci. 13, 221–235 (2008).
Hicks, M., Hing, A., Gao, L., Ryan, J. & Macdonald, P. S. Organ preservation. Methods Mol. Biol. 333, 331–374 (2006).
Maathuis, M. H., Leuvenink, H. G. & Ploeg, R. J. Perspectives in organ preservation. Transplantation 83, 1289–1298 (2007).
Hubert, T. et al. Influence of preservation solution on human islet isolation outcome. Transplantation 83, 270–276 (2007).
Giraud, S. et al. Contribution of large pig for renal ischemia-reperfusion and transplantation studies: the preclinical model. J. Biomed. Biotechnol. 2011, 532127 (2011).
Hauet, T. & Eugene, M. A new approach in organ preservation: potential role of new polymers. Kidney Int. 74, 998–1003 (2008).
Brunkhorst, F. M. & Oppert, M. Nephrotoxicity of hydroxyethyl starch solution. Br. J. Anaesth. 100, 856 (2008).
Bradley, A. J. & Scott, M. D. Immune complex binding by immunocamouflaged [poly(ethylene glycol)-grafted] erythrocytes. Am. J. Hematol. 82, 970–975 (2007).
Le, Y. & Scott, M. D. Immunocamouflage: the biophysical basis of immunoprotection by grafted methoxypoly(ethylene glycol) (mPEG). Acta Biomater. 6, 2631–2641 (2010).
Murad, K. L., Gosselin, E. J., Eaton, J. W. & Scott, M. D. Stealth cells: prevention of major histocompatibility complex class II-mediated T-cell activation by cell surface modification. Blood 94, 2135–2141 (1999).
Eugene, M. Polyethyleneglycols and immunocamouflage of the cells tissues and organs for transplantation. Cell. Mol. Biol. (Noisy-le-grand) 50, 209–215 (2004).
Giraud, S. et al. A new preservation solution increases islet yield and reduces graft immunogenicity in pancreatic islet transplantation. Transplantation 83, 1397–1400 (2007).
Alijani, M. R. et al. Single-donor cold storage versus machine perfusion in cadaver kidney preservation. Transplantation 40, 659–661 (1985).
Balupuri, S. et al. The trouble with kidneys derived from the non heart-beating donor: a single center 10-year experience. Transplantation 69, 842–846 (2000).
Kwiatkowski, A. et al. The early and long term function and survival of kidney allografts stored before transplantation by hypothermic pulsatile perfusion. A prospective randomized study. Ann. Transplant. 14, 14–17 (2009).
Kwiatkowski, A. et al. Machine perfusion preservation improves renal allograft survival. Am. J. Transplant. 7, 1942–1947 (2007).
Moustafellos, P. et al. The influence of pulsatile preservation in kidney transplantation from non-heart-beating donors. Transplant. Proc. 39, 1323–1325 (2007).
Reznik, O. N. et al. Machine perfusion as a tool to select kidneys recovered from uncontrolled donors after cardiac death. Transplant. Proc. 40, 1023–1026 (2008).
Wight, J. P., Chilcott, J. B., Holmes, M. W. & Brewer, N. Pulsatile machine perfusion vs. cold storage of kidneys for transplantation: a rapid and systematic review. Clin. Transplant. 17, 293–307 (2003).
Treckmann, J. et al. Function and quality of kidneys after cold storage, machine perfusion, or retrograde oxygen persufflation: results from a porcine autotransplantation model. Cryobiology 59, 19–23 (2009).
Hosgood, S. A., Yang, B., Bagul, A., Mohamed, I. H. & Nicholson, M. L. A comparison of hypothermic machine perfusion versus static cold storage in an experimental model of renal ischemia reperfusion injury. Transplantation 89, 830–837 (2010).
Irish, W. D. & Katz, E. Cold machine perfusion or static cold storage of kidneys: why the debate continues. Am. J. Transplant. 10, 1955–1956 (2010).
Reich, D. J. et al. ASTS recommended practice guidelines for controlled donation after cardiac death organ procurement and transplantation. Am. J. Transplant. 9, 2004–2011 (2009).
Watson, C. J. et al. Cold machine perfusion versus static cold storage of kidneys donated after cardiac death: a UK multicenter randomized controlled trial. Am. J. Transplant. 10, 1991–1999 (2010).
Jochmans, I. et al. Machine perfusion versus cold storage for the preservation of kidneys donated after cardiac death: a multicenter, randomized, controlled trial. Ann. Surg. 252, 756–764 (2010).
Treckmann, J. et al. Machine perfusion versus cold storage for preservation of kidneys from expanded criteria donors after brain death. Transpl. Int. 24, 548–554 (2011).
Moers, C. et al. Machine perfusion or cold storage in deceased-donor kidney transplantation. N. Engl. J. Med. 360, 7–19 (2009).
Kwiatkowski, A. et al. Influence of preservation method on histopathological lesions of kidney allografts. Ann. Transplant. 14, 10–13 (2009).
Rauen, U. & de Groot, H. New insights into the cellular and molecular mechanisms of cold storage injury. J. Investig. Med. 52, 299–309 (2004).
Brasile, L. et al. Hypothermia—a limiting factor in using warm ischemically damaged kidneys. Am. J. Transplant. 1, 316–320 (2001).
Gage, F. et al. Room temperature pulsatile perfusion of renal allografts with Lifor compared with hypothermic machine pump solution. Transplant. Proc. 41, 3571–3574 (2009).
van de Kerkhove, M. P. et al. Subnormothermic preservation maintains viability and function in a porcine hepatocyte culture model simulating bioreactor transport. Cell Transplant. 15, 161–168 (2006).
Maathuis, M. H. et al. Improved kidney graft function after preservation using a novel hypothermic machine perfusion device. Ann. Surg. 246, 982–988 (2007).
Regner, K. R. et al. Protective effect of Lifor solution in experimental renal ischemia-reperfusion injury. J. Surg. Res. 164, e291–e297 (2010).
Bagul, A. et al. Experimental renal preservation by normothermic resuscitation perfusion with autologous blood. Br. J. Surg. 95, 111–118 (2008).
Hosgood, S. A. & Nicholson, M. L. First in man renal transplantation after ex vivo normothermic perfusion. Transplantation 92, 735–738 (2011).
US National Library of Medicine. ClinicalTrials.gov [online], (2011).
Giraud, S. et al. Direct thrombin inhibitor prevents delayed graft function in a porcine model of renal transplantation. Transplantation 87, 1636–1644 (2009).
Favreau, F. et al. Anti-thrombin therapy during warm ischemia and cold preservation prevents chronic kidney graft fibrosis in a DCD model. Am. J. Transplant. 10, 30–39 (2010).
Thuillier, R. et al. Thrombin inhibition during kidney ischemia-reperfusion reduces chronic graft inflammation and tubular atrophy. Transplantation 90, 612–621 (2010).
Cheadle, C. et al. Effects of anti-adhesive therapy on kidney biomarkers of ischemia reperfusion injury in human deceased donor kidney allografts. Clin. Transplant. 25, 766–775 (2011).
Jayle, C. et al. Protective role of selectin ligand inhibition in a large animal model of kidney ischemia–reperfusion injury. Kidney Int. 69, 1749–1755 (2006).
Rong, S. et al. The TIM-1–TIM-4 pathway enhances renal ischemia-reperfusion injury. J. Am. Soc. Nephrol. 22, 484–495 (2011).
Lewis, A. G., Kohl, G., Ma, Q., Devarajan, P. & Köhl, J. Pharmacological targeting of C5a receptors during organ preservation improves kidney graft survival. Clin. Exp. Immunol. 153, 117–126 (2008).
Davis, A. E. 3rd, Lu, F. & Mejia, P. C1 inhibitor, a multi-functional serine protease inhibitor. Thromb. Haemost. 104, 886–893 (2010).
Castellano, G. et al. Therapeutic targeting of classical and lectin pathways of complement protects from ischemia–reperfusion-induced renal damage. Am. J. Pathol. 176, 1648–1659 (2010).
Fantini, E., Demaison, L., Sentex, E., Grynberg, A. & Athias, P. Some biochemical aspects of the protective effect of trimetazidine on rat cardiomyocytes during hypoxia and reoxygenation. J. Mol. Cell. Cardiol. 26, 949–958 (1994).
Ancerewicz, J. et al. Structure-property relationships of trimetazidine derivatives and model compounds as potential antioxidants. Free Radic. Biol. Med. 25, 113–120 (1998).
Reymond, F. et al. The pH-partition profile of the anti-ischemic drug trimetazidine may explain its reduction of intracellular acidosis. Pharm. Res. 16, 616–624 (1999).
Goujon, J. M., Vandewalle, A., Baumert, H., Carretier, M. & Hauet, T. Influence of cold-storage conditions on renal function of autotransplanted large pig kidneys. Kidney Int. 58, 838–850 (2000).
Hauet, T. et al. Trimetazidine reduces renal dysfunction by limiting the cold ischemia/reperfusion injury in autotransplanted pig kidneys. J. Am. Soc. Nephrol. 11, 138–148 (2000).
Martins, G. F. et al. Trimetazidine on ischemic injury and reperfusion in coronary artery bypass grafting. Arq. Bras. Cardiol. 97, 209–216 (2011).
Matsumoto, S. & Kuroda, Y. Perfluorocarbon for organ preservation before transplantation. Transplantation 74, 1804–1809 (2002).
Kuroda, Y. et al. A new, simple method for cold storage of the pancreas using perfluorochemical. Transplantation 46, 457–460 (1988).
Fujino, Y. et al. Preservation of canine pancreas for 96 hours by a modified two-layer (UW solution/perfluorochemical) cold storage method. Transplantation 51, 1133–1135 (1991).
Yoshikawa, T. et al. Detailed analysis of mucosal restoration of the small intestine after the cavitary two-layer cold storage method. Am. J. Transplant. 5, 2135–2142 (2005).
Hosgood, S. A., Mohamed, I. H. & Nicholson, M. L. The two layer method does not improve the preservation of porcine kidneys. Med. Sci. Monit. 17, BR27–BR33 (2011).
Hosgood, S. A., Nicholson, H. F. & Nicholson, M. L. Oxygenated kidney preservation techniques. Transplantation 93, 455–459 (2012).
Kakehata, J. et al. Therapeutic potentials of an artificial oxygen-carrier, liposome-encapsulated hemoglobin, for ischemia/reperfusion-induced cerebral dysfunction in rats. J. Pharmacol. Sci. 114, 189–197 (2010).
Spahn, D. R. & Kocian, R. Artificial O2 carriers: status in 2005. Curr. Pharm. Des. 11, 4099–4114 (2005).
Thuillier, R. et al. Supplementation with a new therapeutic oxygen carrier reduces chronic fibrosis and organ dysfunction in kidney static preservation. Am. J. Transplant. 11, 1845–1860 (2011).
van der Wouden, E. A., Sandovici, M., Henning, R. H., de Zeeuw, D. & Deelman, L. E. Approaches and methods in gene therapy for kidney disease. J. Pharmacol. Toxicol. Methods 50, 13–24 (2004).
Castanotto, D. & Rossi, J. J. The promises and pitfalls of RNA-interference-based therapeutics. Nature 457, 426–433 (2009).
Stokman, G., Qin, Y., Racz, Z., Hamar, P. & Price, L. S. Application of siRNA in targeting protein expression in kidney disease. Adv. Drug Deliv. Rev. 62, 1378–1389 (2010).
Kahan, B. D. et al. Phase I and phase II safety and efficacy trial of intercellular adhesion molecule-1 antisense oligodeoxynucleotide (ISIS 2302) for the prevention of acute allograft rejection. Transplantation 78, 858–863 (2004).
Ferrari, N. et al. Characterization of antisense oligonucleotides comprising 2'-deoxy-2'-fluoro-β -D-arabinonucleic acid (FANA): specificity, potency, and duration of activity. Ann. NY Acad. Sci. 1082, 91–102 (2006).
Zheng, X. et al. Novel small interfering RNA-containing solution protecting donor organs in heart transplantation. Circulation 120, 1099–1107 (2009).
Yang, B., Hosgood, S. A. & Nicholson, M. L. Naked small interfering RNA of caspase-3 in preservation solution and autologous blood perfusate protects isolated ischemic porcine kidneys. Transplantation 91, 501–507 (2011).
Tsoulfas, G. et al. Hydrodynamic plasmid DNA gene therapy model in liver transplantation. J. Surg. Res. 135, 242–249 (2006).
Kosieradzki, M. et al. Early function of kidneys stored by continuous hypothermic pulsatile perfusion can be predicted using a new “viability index”. Transplant. Proc. 34, 541–543 (2002).
Moers, C. et al. The value of machine perfusion perfusate biomarkers for predicting kidney transplant outcome. Transplantation 90, 966–973 (2010).
Liu, Q. et al. Discriminate liver warm ischemic injury during hypothermic machine perfusion by proton magnetic resonance spectroscopy: a study in a porcine model. Transplant. Proc. 41, 3383–3386 (2009).
Bon, D. et al. NMR analysis of solution during machine perfusion: predicting graft outcome? Abstracts of the 15th Congress of the European Society for Organ Transplantation & 22nd Annual Conference of the British Society for Histocompatibility & Immunogenetics. Glasgow, UK. Transpl. Int. 24 (Suppl. 2), O-033 (2011).
Garfield, S. S. & Evans, R. W. Machine perfusion cost-effectiveness versus cold storage has been demonstrated; limiting use to marginal donor kidneys unjustified. Transpl. Int. 23, e67–e68 (2010).
Wszola, M. et al. Long term medical and economical benefit of machine perfusion (MP) kidney storage in comparison to cold storage (CS). Ann. Transplant. 14, 24–29 (2009).
Szwarc, I. et al. Ischemic postconditioning prevents ischemic acute renal failure. Transplant. Proc. 39, 2554–2556 (2007).
Chen, H. et al. Ischemic postconditioning inhibits apoptosis after renal ischemia/reperfusion injury in rat. Transpl. Int. 21, 364–371 (2008).
Eldaif, S. M. et al. Attenuation of renal ischemia-reperfusion injury by postconditioning involves adenosine receptor and protein kinase C activation. Transpl. Int. 23, 217–226 (2010).
Kaur, S., Jaggi, A. S. & Singh, N. Molecular aspects of ischaemic postconditioning. Fundam. Clin. Pharmacol. 23, 521–536 (2009).
Penna, C., Mancardi, D., Raimondo, S., Geuna, S. & Pagliaro, P. The paradigm of postconditioning to protect the heart. J. Cell. Mol. Med. 12, 435–458 (2008).
Dal Ponte, C. et al. Pharmacological postconditioning protects against hepatic ischemia/reperfusion injury. Liver Transpl. 17, 474–482 (2011).
US National Library of Medicine. ClinicalTrials.gov [online], (2011).
Ritter, T., Nosov, M. & Griffin, M. D. Gene therapy in transplantation: toward clinical trials. Curr. Opin. Mol. Ther. 11, 504–512 (2009).
Molitoris, B. A. et al. siRNA targeted to p53 attenuates ischemic and cisplatin-induced acute kidney injury. J. Am. Soc. Nephrol. 20, 1754–1764 (2009).
Li, N. et al. Adjuvant adenovirus-mediated delivery of herpes simplex virus thymidine kinase administration improves outcome of liver transplantation in patients with advanced hepatocellular carcinoma. Clin. Cancer Res. 13, 5847–5854 (2007).
Benigni, A., Morigi, M. & Remuzzi, G. Kidney regeneration. Lancet 375, 1310–1317 (2011).
Humphreys, B. D. & Bonventre, J. V. The contribution of adult stem cells to renal repair. Nephrol. Ther. 3, 3–10 (2007).
Valina, C. et al. Intracoronary administration of autologous adipose tissue-derived stem cells improves left ventricular function, perfusion, and remodelling after acute myocardial infarction. Eur. Heart J. 28, 2667–2677 (2007).
Mazo, M. et al. Transplantation of adipose derived stromal cells is associated with functional improvement in a rat model of chronic myocardial infarction. Eur. J. Heart Fail. 10, 454–462 (2008).
Kim, Y. et al. Direct comparison of human mesenchymal stem cells derived from adipose tissues and bone marrow in mediating neovascularization in response to vascular ischemia. Cell. Physiol. Biochem. 20, 867–876 (2007).
Feng, Z. et al. Fresh and cryopreserved, uncultured adipose tissue-derived stem and regenerative cells ameliorate ischemia-reperfusion-induced acute kidney injury. Nephrol. Dial. Transplant. 25, 3874–3884 (2010).
Baer, P. C. Adipose-derived stem cells and their potential to differentiate into the epithelial lineage. Stem Cells Dev. 20, 1805–1816 (2011).
Hara, Y. et al. In vivo effect of bone marrow-derived mesenchymal stem cells in a rat kidney transplantation model with prolonged cold ischemia. Transpl. Int. 24, 1112–1123 (2011).
De Coppi, P. et al. Isolation of amniotic stem cell lines with potential for therapy. Nat. Biotechnol. 25, 100–106 (2007).
Trounson, A. A fluid means of stem cell generation. Nat. Biotechnol. 25, 62–63 (2007).
Blotiere, P. O., Tuppin, P., Weill, A., Ricordeau, P. & Allemand, H. The cost of dialysis and kidney transplantation in France in 2007, impact of an increase of peritoneal dialysis and transplantation [French]. Nephrol. Ther. 6, 240–247 (2010).
Author information
Authors and Affiliations
Contributions
All the authors researched data for the article, contributed to the discussion of content and wrote the article. D. Bon and T. Hauet reviewed and/or edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
T. Hauet is an independent inventor and holder of European patent EP1997374 with MacoPharma.
Rights and permissions
About this article
Cite this article
Bon, D., Chatauret, N., Giraud, S. et al. New strategies to optimize kidney recovery and preservation in transplantation. Nat Rev Nephrol 8, 339–347 (2012). https://doi.org/10.1038/nrneph.2012.83
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrneph.2012.83
This article is cited by
-
Evaluation of the efficacy of HEMO2life®, a marine OXYgen carrier for Organ Preservation (OxyOp2) in renal transplantation: study protocol for a multicenter randomized trial
Trials (2023)
-
Combination of mesenchymal stromal cells and machine perfusion is a novel strategy for organ preservation in solid organ transplantation
Cell and Tissue Research (2021)
-
Protection of the transplant kidney during cold perfusion with doxycycline: proteomic analysis in a rat model
Proteome Science (2020)
-
Renal effects of exendin-4 in an animal model of brain death
Molecular Biology Reports (2019)
-
Changes in the metabolic composition of storage solution with prolonged cold ischemia of the uterus
Journal of Assisted Reproduction and Genetics (2019)