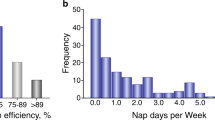
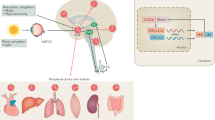
Abstract
Sleep and circadian rhythm disruption are frequently observed in patients with psychiatric disorders and neurodegenerative disease. The abnormal sleep that is experienced by these patients is largely assumed to be the product of medication or some other influence that is not well defined. However, normal brain function and the generation of sleep are linked by common neurotransmitter systems and regulatory pathways. Disruption of sleep alters sleep–wake timing, destabilizes physiology and promotes a range of pathologies (from cognitive to metabolic defects) that are rarely considered to be associated with abnormal sleep. We propose that brain disorders and abnormal sleep have a common mechanistic origin and that many co-morbid pathologies that are found in brain disease arise from a destabilization of sleep mechanisms. The stabilization of sleep may be a means by which to reduce the symptoms of — and permit early intervention of — psychiatric and neurodegenerative disease.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Kraeplin, E. Compendium der Psychiatrie zum Gebrauch fuer Studierende und Aerzte (Abel Verlag, Leipzig, 1883).
Papousek, M. [Chronobiological aspects of cyclothymia (author's transl)]. Fortschr. Neurol. Psychiatr. Grenzgeb. 43, 381–440 (1975).
Wehr, T. A., Sack, D., Rosenthal, N., Duncan, W. & Gillin, J. C. Circadian rhythm disturbances in manic-depressive illness. Fed. Proc. 42, 2809–2814 (1983).
Wirz-Justice, A., Puhringer, W. & Hole, G. Sleep deprivation and clomipramine in endogenous depression. Lancet 2, 912 (1976).
Wirz-Justice, A. et al. Sleep deprivation: effects on circadian rhythms of rat brain neurotransmitter receptors. Psychiatry Res. 5, 67–76 (1981).
Cirelli, C. The genetic and molecular regulation of sleep: from fruit flies to humans. Nature Rev. Neurosci. 10, 549–560 (2009).
Wirz-Justice, A. Biological rhythm disturbances in mood disorders. Int. Clin. Psychopharmacol. 21, (Suppl. 1) 11–15 (2006).
Wirz-Justice, A., Haug, H. J. & Cajochen, C. Disturbed circadian rest–activity cycles in schizophrenia patients: an effect of drugs? Schizophr. Bull. 27, 497–502 (2001).
Smith, M. T., Perlis, M. L., Smith, M. S., Giles, D. E. & Carmody, T. P. Sleep quality and presleep arousal in chronic pain. J. Behav. Med. 23, 1–13 (2000).
Takahashi, J. S., Hong, H. K., Ko, C. H. & McDearmon, E. L. The genetics of mammalian circadian order and disorder: implications for physiology and disease. Nature Rev. Genet. 9, 764–775 (2008).
Mieda, M. & Sakurai, T. Integrative physiology of orexins and orexin receptors. CNS Neurol. Disord. Drug Targets 8, 281–295 (2009).
Feder, A., Nestler, E. J. & Charney, D. S. Psychobiology and molecular genetics of resilience. Nature Rev. Neurosci. 10, 446–457 (2009).
Wulff, K., Porcheret, K., Cussans, E. & Foster, R. G. Sleep and circadian rhythm disturbances: multiple genes and multiple phenotypes. Curr. Opin. Genet. Dev. 19, 237–246 (2009).
Armitage, R. Sleep and circadian rhythms in mood disorders. Acta Psychiatr. Scand. Suppl. 115, 104–115 (2007).
Schibler, U. The daily timing of gene expression and physiology in mammals. Dialogues Clin. Neurosci. 9, 257–272 (2007).
Katz, G., Durst, R., Zislin, Y., Barel, Y. & Knobler, H. Y. Psychiatric aspects of jet lag: review and hypothesis. Med. Hypotheses 56, 20–23 (2001).
Benedetti, F., Barbini, B., Colombo, C. & Smeraldi, E. Chronotherapeutics in a psychiatric ward. Sleep Med. Rev. 11, 509–522 (2007).
Wirz-Justice, A. & Van den Hoofdakker, R. H. Sleep deprivation in depression: what do we know, where do we go? Biol. Psychiatry 46, 445–453 (1999).
Bunney, J. N. & Potkin, S. G. Circadian abnormalities, molecular clock genes and chronobiological treatments in depression. Br. Med. Bull. 86, 23–32 (2008).
Pigeon, W. R. et al. Is insomnia a perpetuating factor for late-life depression in the IMPACT cohort? Sleep 31, 481–488 (2008).
Posmontier, B. Sleep quality in women with and without postpartum depression. J. Obstet. Gynecol. Neonatal Nurs. 37, 722–735; (2008).
Asnis, G. M. et al. Zolpidem for persistent insomnia in SSRI-treated depressed patients. J. Clin. Psychiatry 60, 668–676 (1999).
Krystal, A. D., Thakur, M. & Roth, T. Sleep disturbance in psychiatric disorders: effects on function and quality of life in mood disorders, alcoholism, and schizophrenia. Ann. Clin. Psychiatry 20, 39–46 (2008).
Kasper, S. et al. Efficacy of the novel antidepressant agomelatine on the circadian rest-activity cycle and depressive and anxiety symptoms in patients with major depressive disorder: a randomized, double-blind comparison with sertraline. J. Clin. Psychiatry 71, 109–120 (2010).
Lopes, M. C., Quera-Salva, M. A. & Guilleminault, C. Non-REM sleep instability in patients with major depressive disorder: subjective improvement and improvement of non-REM sleep instability with treatment (Agomelatine). Sleep Med. 9, 33–41 (2007).
Olie, J. P. & Kasper, S. Efficacy of agomelatine, a MT1/MT2 receptor agonist with 5-HT2C antagonistic properties, in major depressive disorder. Int. J. Neuropsychopharmacol. 10, 661–673 (2007).
Quera-Salva, M. A., Lemoine, P. & Guilleminault, C. Impact of the novel antidepressant agomelatine on disturbed sleep–wake cycles in depressed patients. Hum. Psychopharmacol. 25, 222–229 (2010).
Fava, M. et al. Modafinil augmentation of selective serotonin reuptake inhibitor therapy in MDD partial responders with persistent fatigue and sleepiness. Ann. Clin. Psychiatry 19, 153–159 (2007).
Kupfer, D. J. et al. REM sleep, naps, and depression. Psychiatry Res. 5, 195–203 (1981).
Modell, S., Huber, J., Holsboer, F. & Lauer, C. J. The munich vulnerability study on affective disorders: risk factors for unipolarity versus bipolarity. J. Affect. Disord. 74, 173–184 (2003).
Rao, U. et al. Heterogeneity in EEG sleep findings in adolescent depression: unipolar versus bipolar clinical course. J. Affect. Disord. 70, 273–280 (2002).
Gregory, A. M., Cox, J., Crawford, M. R., Holland, J. & Haravey, A. G. Dysfunctional beliefs and attitudes about sleep in children. J. Sleep Res. 18, 422–426 (2009).
Levitt, A. J. & Boyle, M. H. The impact of latitude on the prevalence of seasonal depression. Can. J. Psychiatry 47, 361–367 (2002).
Axelsson, J., Ragnarsdottir, S., Pind, J. & Sigbjornsson, R. Daylight availability: a poor predictor of depression in Iceland. Int. J. Circumpolar Health 63, 267–276 (2004).
Wirz-Justice, A. Chronobiology and psychiatry. Sleep Med. Rev. 11, 423–427 (2007).
Lavoie, M. P. et al. Evidence of a biological effect of light therapy on the retina of patients with seasonal affective disorder. Biol. Psychiatry 66, 253–258 (2009).
Hankins, M. W., Peirson, S. N. & Foster, R. G. Melanopsin: an exciting photopigment. Trends Neurosci. 31, 27–36 (2008).
McClung, C. A. Circadian genes, rhythms and the biology of mood disorders. Pharmacol. Ther. 114, 222–232 (2007).
Plante, D. T. & Winkelman, J. W. Sleep disturbance in bipolar disorder: therapeutic implications. Am. J. Psychiatry 165, 830–843 (2008).
Young, A. H. Antiglucocoticoid treatments for depression. Aust. N. Z. J. Psychiatry 40, 402–405 (2006).
Roybal, K. et al. Mania-like behavior induced by disruption of CLOCK. Proc. Natl Acad. Sci. USA 104, 6406–6411 (2007).
Salvatore, P. et al. Circadian activity rhythm abnormalities in ill and recovered bipolar I disorder patients. Bipolar Disord. 10, 256–265 (2008).
Benedetti, F. et al. A length polymorphism in the circadian clock gene Per3 influences age at onset of bipolar disorder. Neurosci. Lett. 445, 184–187 (2008).
Bellivier, F. et al. Age at onset in bipolar I affective disorder: further evidence for three subgroups. Am. J. Psychiatry 160, 999–1001 (2003).
Conus, P. et al. The proximal prodrome to first episode mania — a new target for early intervention. Bipolar Disord. 10, 555–565 (2008).
Modell, S., Ising, M., Holsboer, F. & Lauer, C. J. The Munich vulnerability study on affective disorders: premorbid polysomnographic profile of affected high-risk probands. Biol. Psychiatry 58, 694–699 (2005).
Roehrs, T. & Roth, T. Sleep, sleepiness, sleep disorders and alcohol use and abuse. Sleep Med. Rev. 5, 287–297 (2001).
Krystal, A. D. Treating the health, quality of life, and functional impairments in insomnia. J. Clin. Sleep Med. 3, 63–72 (2007).
Hatonen, T., Forsblom, S., Kieseppa, T., Lonnqvist, J. & Partonen, T. Circadian phenotype in patients with the co-morbid alcohol use and bipolar disorders. Alcohol Alcohol. 43, 564–568 (2008).
Spanagel, R. et al. The clock gene Per2 influences the glutamatergic system and modulates alcohol consumption. Nature Med. 11, 35–42 (2005).
Franken, P., Thomason, R., Heller, H. C. & O'Hara, B. F. A non-circadian role for clock-genes in sleep homeostasis: a strain comparison. BMC Neurosci. 8, 87 (2007).
Rodd, Z. A. et al. Candidate genes, pathways and mechanisms for alcoholism: an expanded convergent functional genomics approach. Pharmacogenomics J. 7, 222–256 (2007).
Francks, C. et al. Population-based linkage analysis of schizophrenia and bipolar case-control cohorts identifies a potential susceptibility locus on 19q13. Mol. Psychiatry 15, 319–325 (2008).
Hendler, T., Bleich-Cohen, M. & Sharon, H. Neurofunctional view of psychiatry: clinical brain imaging revisited. Curr. Opin. Psychiatry 22, 300–305 (2009).
Stahl, S. M. & Buckley, P. F. Negative symptoms of schizophrenia: a problem that will not go away. Acta Psychiatr. Scand. 115, 4–11 (2007).
Van Cauter, E. et al. Circadian and sleep-related endocrine rhythms in schizophrenia. Arch Gen Psychiatry 48, 348–356 (1991).
Cohrs, S. Sleep disturbances in patients with schizophrenia: impact and effect of antipsychotics. CNS Drugs 22, 939–962 (2008).
Auslander, L. A. & Jeste, D. V. Perceptions of problems and needs for service among middle-aged and elderly outpatients with schizophrenia and related psychotic disorders. Community Ment. Health J. 38, 391–402 (2002).
Hofstetter, J. R., Lysaker, P. H. & Mayeda, A. R. Quality of sleep in patients with schizophrenia is associated with quality of life and coping. BMC Psychiatry 5, 13 (2005).
Tomppo, L. et al. Association of variants in DISC1 with psychosis-related traits in a large population cohort. Arch. Gen. Psychiatry 66, 134–141 (2009).
Sawamura, N. et al. Nuclear DISC1 regulates CRE-mediated gene transcription and sleep homeostasis in the fruit fly. Mol. Psychiatry 13, 1138–1148 (2008).
Gottlieb, D. J., O'Connor, G. T. & Wilk, J. B. Genome-wide association of sleep and circadian phenotypes. BMC Med. Genet. 8, (Suppl. 1) 9 (2007).
Tomppo, L. et al. Association between genes of disrupted in schizophrenia 1 (DISC1) interactors and schizophrenia supports the role of the DISC1 pathway in the etiology of major mental illnesses. Biol. Psychiatry 65, 1055–1062 (2009).
Takao, T., Tachikawa, H., Kawanishi, Y., Mizukami, K. & Asada, T. CLOCK gene T3111C polymorphism is associated with Japanese schizophrenics: a preliminary study. Eur. Neuropsychopharmacol. 17, 3273–3276 (2007).
Ding, J. M. et al. Resetting the biological clock: mediation of nocturnal circadian shifts by glutamate and NO. Science 266, 1713–1717 (1994).
Young, C. E. et al. SNAP-25 deficit and hippocampal connectivity in schizophrenia. Cereb. Cortex 8, 261–268 (1998).
Oliver, P. L. & Davies, K. E. Interaction between environmental and genetic factors modulates schizophrenic endophenotypes in the Snap-25 mouse mutant blind–drunk. Hum. Mol. Genet. 18, 4576–4589 (2009).
Deery, M. J. et al. Proteomic analysis reveals the role of synaptic vesicle cycling in sustaining the suprachiasmatic circadian clock. Curr. Biol. 19, 2031–2036 (2009).
Monti, J. M. & Monti, D. Sleep disturbance in generalized anxiety disorder and its treatment. Sleep Med. Rev. 4, 263–276 (2000).
Papadimitriou, G. N. & Linkowski, P. Sleep disturbance in anxiety disorders. Int. Rev. Psychiatry 17, 229–236 (2005).
Xu, Y. L. et al. Neuropeptide S: a neuropeptide promoting arousal and anxiolytic-like effects. Neuron 43, 487–497 (2004).
Reinscheid, R. K. & Xu, Y. L. Neuropeptide S as a novel arousal promoting peptide transmitter. FEBS J. 272, 5689–5693 (2005).
Okamura, N. & Reinscheid, R. K. Neuropeptide S: a novel modulator of stress and arousal. Stress 10, 221–226 (2007).
Leonard, S. K. et al. Pharmacology of neuropeptide S. in mice: therapeutic relevance to anxiety disorders. Psychopharmacology (Berlin) 197, 601–611 (2008).
Reinscheid, R. K. Neuropeptide S: anatomy, pharmacology, genetics and physiological functions. Results Probl. Cell Differ. 46, 145–158 (2008).
Okamura, N. et al. Gender-specific association of a functional coding polymorphism in the Neuropeptide S receptor gene with panic disorder but not with schizophrenia or attention-deficit/hyperactivity disorder. Prog. Neuropsychopharmacol. Biol. Psychiatry 31, 1444–1448 (2007).
Bohnen, N. I. & Albin, R. L. The cholinergic system and Parkinson disease. Behav. Brain Res. 7 Jan 2010 (doi:10.1016/j.bbr.2009.12.048).
Jeong, J. EEG dynamics in patients with Alzheimer's disease. Clin. Neurophysiol. 115, 1490–1505 (2004).
Hofman, M. A. & Swaab, D. F. Alterations in circadian rhythmicity of the vasopressin-producing neurons of the human suprachiasmatic nucleus (SCN) with aging. Brain Res. 651, 134–142 (1994).
Vitiello, M. V., Prinz, P. N., Williams, D. E., Frommlet, M. S. & Ries, R. K. Sleep disturbances in patients with mild-stage Alzheimer's disease. J. Gerontol. 45, M131–M138 (1990).
Clemens, Z., Fabo, D. & Halasz, P. Overnight verbal memory retention correlates with the number of sleep spindles. Neuroscience 132, 529–535 (2005).
Tractenberg, R. E., Singer, C. M. & Kaye, J. A. Characterizing sleep problems in persons with Alzheimer's disease and normal elderly. J. Sleep Res. 15, 97–103 (2006).
Zhou, J. N., Liu, R. Y., Kamphorst, W., Hofman, M. A. & Swaab, D. F. Early neuropathological Alzheimer's changes in aged individuals are accompanied by decreased cerebrospinal fluid melatonin levels. J. Pineal Res. 35, 125–130 (2003).
Rajaratnam, S. M. & Arendt, J. Health in a 24-h society. Lancet 358, 999–1005 (2001).
Dowling, G. A. et al. Melatonin and bright-light treatment for rest-activity disruption in institutionalized patients with Alzheimer's disease. J. Am. Geriatr. Soc. 56, 239–246 (2008).
Parkinson, J. An essay on the shaking palsy. 1817. J. Neuropsychiatry Clin. Neurosci. 14, 223–236 (2002); discussion in 14, 222 (2002).
Arnulf, I., Leu, S. & Oudiette, D. Abnormal sleep and sleepiness in Parkinson's disease. Curr. Opin. Neurol. 21, 472–477 (2008).
Willis, G. L., Kelly, A. M. & Kennedy, G. A. Compromised circadian function in Parkinson's disease: enucleation augments disease severity in the unilateral model. Behav. Brain Res. 193, 37–47 (2008).
Metzler-Baddeley, C. A review of cognitive impairments in dementia with Lewy bodies relative to Alzheimer's disease and Parkinson's disease with dementia. Cortex 43, 583–600 (2007).
Kassubek, J. et al. Topography of cerebral atrophy in early Huntington's disease: a voxel based morphometric MRI study. J. Neurol. Neurosurg. Psychiatry 75, 213–220 (2004).
Rosas, H. D. et al. Evidence for more widespread cerebral pathology in early HD: an MRI-based morphometric analysis. Neurology 60, 1615–1620 (2003).
Morton, A. J. et al. Disintegration of the sleep–wake cycle and circadian timing in Huntington's disease. J. Neurosci. 25, 157–163 (2005).
Goodman, A. O. & Barker, R. A. How vital is sleep in Huntington's disease? J. Neurol. 257, 882–897 (2010).
Pallier, P. N. et al. Pharmacological imposition of sleep slows cognitive decline and reverses dysregulation of circadian gene expression in a transgenic mouse model of Huntington's disease. J. Neurosci. 27, 7869–7878 (2007).
Pallier, P. N. & Morton, A. J. Management of sleep/wake cycles improves cognitive function in a transgenic mouse model of Huntington's disease. Brain Res. 1279, 90–98 (2009).
Taphoorn, M. J. et al. Fatigue, sleep disturbances and circadian rhythm in multiple sclerosis. J. Neurol. 240, 446–448 (1993).
Attarian, H. P., Brown, K. M., Duntley, S. P., Carter, J. D. & Cross, A. H. The relationship of sleep disturbances and fatigue in multiple sclerosis. Arch. Neurol. 61, 525–528 (2004).
Gallup, A. C., Gallup, G. G. Jr. & Feo, C. Yawning, sleep, and symptom relief in patients with multiple sclerosis. Sleep Med. 11, 329–330 (2010).
Mendozzi, L., Tronci, F., Garegnani, M. & Pugnetti, L. Sleep disturbance and fatigue in mild relapsing remitting multiple sclerosis patients on chronic immunomodulant therapy: an actigraphic study. Mult. Scler. 16, 238–247 (2010).
Ceccarelli, A. et al. T2 hypointensity in the deep gray matter of patients with benign multiple sclerosis. Mult. Scler. 15, 678–686 (2009).
Auer, R. N., Rowlands, C. G., Perry, S. F. & Remmers, J. E. Multiple sclerosis with medullary plaques and fatal sleep apnea (Ondine's curse). Clin. Neuropathol. 15, 101–105 (1996).
Wirz-Justice, A., Benedetti, F. & Terman, M. Chronotherapeutics for Affective Disorders (Karger, Basel, 2009).
Damiola, F. et al. Restricted feeding uncouples circadian oscillators in peripheral tissues from the central pacemaker in the suprachiasmatic nucleus. Genes Dev. 14, 2950–2961 (2000).
Goetz, F. et al. Timing of single daily meal influences relations among human circadian rhythms in urinary cyclic AMP and hemic glucagon, insulin and iron. Experientia 32, 1081–1084 (1976).
Riemann, D. et al. The hyperarousal model of insomnia: a review of the concept and its evidence. Sleep Med. Rev. 14, 19–31 (2010).
Pace-Schott, E. F. & Hobson, J. A. The neurobiology of sleep: genetics, cellular physiology and subcortical networks. Nature Rev. Neurosci. 3, 591–605 (2002).
Diniz Behn, C. G., Kopell, N., Brown, E. N., Mochizuki, T. & Scammell, T. E. Delayed orexin signaling consolidates wakefulness and sleep: physiology and modeling. J. Neurophysiol. 99, 3090–3103 (2008).
Behn, C. G., Brown, E. N., Scammell, T. E. & Kopell, N. J. Mathematical model of network dynamics governing mouse sleep-wake behavior. J. Neurophysiol. 97, 3828–3840 (2007).
Lockley, S. W. et al. Short-wavelength sensitivity for the direct effects of light on alertness, vigilance, and the waking electroencephalogram in humans. Sleep 29, 161–168 (2006).
Scheer, F. A., Wright, K. P. Jr, Kronauer, R. E. & Czeisler, C. A. Plasticity of the intrinsic period of the human circadian timing system. PLoS ONE 2, e721 (2007).
Meijer, J. H., Michel, S. & Vansteensel, M. J. Processing of daily and seasonal light information in the mammalian circadian clock. Gen. Comp. Endocrinol. 152, 159–164 (2007).
Buijs, R. M., van Eden, C. G., Goncharuk, V. D. & Kalsbeek, A. The biological clock tunes the organs of the body: timing by hormones and the autonomic nervous system. J. Endocrinol. 177, 17–26 (2003).
Lupi, D., Oster, H., Thompson, S. & Foster, R. G. The acute light-induction of sleep is mediated by OPN4-based photoreception. Nature Neurosci. 11, 1068–1073 (2008).
Ko, C. H. & Takahashi, J. S. Molecular components of the mammalian circadian clock. Hum. Mol. Genet. 15, R271–R277 (2006).
Schibler, U. The 2008 Pittendrigh/Aschoff lecture: peripheral phase coordination in the mammalian circadian timing system. J. Biol. Rhythms 24, 3–15 (2009).
Corradini, I., Verderio, C., Sala, M., Wilson, M. C. & Matteoli, M. SNAP-25 in neuropsychiatric disorders. Ann. N. Y. Acad. Sci. 1152, 93–99 (2009).
Yi, C. X. et al. Ventromedial arcuate nucleus communicates peripheral metabolic information to the suprachiasmatic nucleus. Endocrinology 147, 283–294 (2006).
Malek, Z. S., Sage, D., Pevet, P. & Raison, S. Daily rhythm of tryptophan hydroxylase-2 messenger ribonucleic acid within raphe neurons is induced by corticoid daily surge and modulated by enhanced locomotor activity. Endocrinology 148, 5165–5172 (2007).
He, Y. et al. The transcriptional repressor DEC2 regulates sleep length in mammals. Science 325, 866–870 (2009).
Maywood, E. S., O'Neill, J. S., Chesham, J. E. & Hastings, M. H. Minireview: the circadian clockwork of the suprachiasmatic nuclei — analysis of a cellular oscillator that drives endocrine rhythms. Endocrinology 148, 5624–5634 (2007).
Godinho, S. I. et al. The after-hours mutant reveals a role for Fbxl3 in determining mammalian circadian period. Science 316, 897–900 (2007).
Dijk, D. J. & Archer, S. N. PERIOD3, circadian phenotypes, and sleep homeostasis. Sleep Med. Rev. 14, 151–160 (2010).
Reghunandanan, V. & Reghunandanan, R. Neurotransmitters of the suprachiasmatic nuclei. J. Circadian Rhythms 4, 2 (2006).
Acknowledgements
The work is supported by the National Institute for Health Research (NIHR). Biomedical Research Centre, Oxford, UK, and The Wellcome Trust, London, UK. We would like to thank G. Goodwin (head of the Department of Psychiatry, University of Oxford, UK), C. Kennard (head of the Department of Clinical Neurology, University of Oxford, UK), K. Porcheret (Nuffield Laboratory of Ophthalmology, University of Oxford, UK), K. Davies and P. Oliver (Medical Research Council Functional Genomics Unit, University of Oxford, UK) for their valuable input during the preparation of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary information S1 (table)
Circadian/sleep-related abnormalities observed in a range of syndromes with some emerging sleep/circadian genetic associations (PDF 441 kb)
Supplementary information S2 (table)
references for Figure 3 on the health consequences of shortened/disrupted sleep and circadian rhythms. (PDF 421 kb)
Related links
Glossary
- Bipolar disorder
-
A disorder characterized by abrupt mixed states of mood from an energetic elevated mood (termed mania or, if milder, hypomania) to a deep depressive state. Bipolar disorder type 1 classification is based on the occurrence of at least one manic episode, with or without the occurrence of a major depressive episode. Bipolar disorder type 2 is characterized by at least one hypomania episode and one major depressive state.
- Chronotype
-
An individual's preference for daytime or night-time activities (also referred to as morningness and eveningness or larks and owls, respectively). Morning types wake up early and are most alert in the first part of the day, whereas evening types are most alert in the late evening hours and prefer to go to bed late.
- Circadian phase
-
A particular reference point in the circadian cycle. For example, the onset of sleep.
- Circadian system
-
(Also known as process C). The entire molecular, cellular and physiological basis for the generation of circadian rhythms in an organism.
- Diagnostic and statistical manual of mental disorders
-
(DSM-IV). A manual published by the American Psychiatric Association that provides diagnostic criteria for mental health disorders. DSM-IV-TR is the most recent, text-revised version published in 2000.
- Diurnal
-
An activity or process that occurs during the daytime (during light).
- Electroencephalography
-
(EEG). A measure of the electrical activity of the brain that can be used to define different wake, NREM and REM sleep states.
- Endophenotype
-
A special type of biomarker. In mental health, it is the division of behavioural symptoms into recognizable phenotypes with a clear genetic association.
- Entrainment
-
The process by which an organism's circadian rhythm is synchronized to an environmental rhythm such as the light–dark cycle.
- Hypothalamic-pituitary-adrenal axis
-
A complex set of direct influences and feedback interactions among the hypothalamus, pituitary gland and adrenal glands. The hypothalamic-pituitary-adrenal (HPA) axis constitutes a major part of the neuroendocrine system that controls reactions to stress.
- International Classification of Disorders
-
(ICD-10). A disease classification published by the World Health Organization that provides diagnostic criteria for mental health disorders. The ICD-10 classification consists of 10 main groups.
- K complexes
-
A brief, negative high-voltage peak, usually greater than 100 μV. Like sleep spindles, K-complexes are another characteristic of stage two sleep.
- Light therapy
-
(Also known as phototherapy). Consists of exposure to daylight or artificial light (provided by a light box). Light exposure is of a defined intensity and is given at a specific time. Light therapy has been used to treat circadian rhythm disorders, such as delayed sleep phase syndrome, and can also be used to treat seasonal affective disorder.
- Major depressive disorder
-
A disorder characterized by severe, highly persistent depression and a loss of interest or pleasure in everyday activities. It is often associated with lack of appetite, chronic fatigue and sleep disturbances. There is an increased risk of suicide.
- Narcolepsy
-
A chronic sleep disorder (or dyssomnia). In relation to sleep, the condition is characterized by excessive daytime sleepiness whereby the individual experiences extreme fatigue at inappropriate times and may fall asleep.
- Non-rapid eye movement sleep
-
(NREM). There are four distinct stages of NREM sleep (NREM 1–4) defined on the basis of EEG or polysomnography and other characteristics that are seen in each stage.
- Prodromal
-
An early symptom (or set of symptoms) that might indicate the start of a disease before specific symptoms occur.
- Selective serotonin reuptake inhibitors
-
(Also known as serotonin-specific reuptake inhibitors). A class of compounds typically used as antidepressants in the treatment of depression, anxiety disorders and some personality disorders. These inhibitors increase the extracellular level of the neurotransmitter serotonin by inhibiting its reuptake into the presynaptic cell. This increases the level of serotonin that is available to bind to the postsynaptic receptor.
- Sleep spindles
-
Stage 2 sleep is characterized by sleep spindles that signify a burst of brain activity which is visible on an electroencephalogram (EEG) ranging from 11 to 16 Hz.
Rights and permissions
About this article
Cite this article
Wulff, K., Gatti, S., Wettstein, J. et al. Sleep and circadian rhythm disruption in psychiatric and neurodegenerative disease. Nat Rev Neurosci 11, 589–599 (2010). https://doi.org/10.1038/nrn2868
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrn2868
This article is cited by
-
Overtime work is related to nonrestorative sleep independently of short sleep time among a Japanese occupational population
International Archives of Occupational and Environmental Health (2024)
-
Causal associations between sleep traits and brain structure: a bidirectional Mendelian randomization study
Behavioral and Brain Functions (2023)
-
Optimizing the methodology of human sleep and memory research
Nature Reviews Psychology (2023)
-
The GABAA receptor modulator zolpidem augments hippocampal-prefrontal coupling during non-REM sleep
Neuropsychopharmacology (2023)
-
Sleep quality in students: Associations with psychological and lifestyle factors
Current Psychology (2023)