Key Points
-
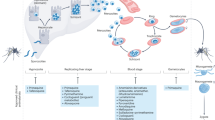
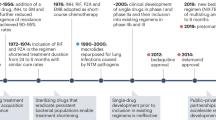
The global tuberculosis (TB) epidemic has not dwindled but has actually grown, largely owing to the challenges that are presented by persistence and resistance. Huge advances in therapeutic regimens are needed to dramatically reduce the course of treatment from months to days.
-
To realize the vulnerabilities of Mycobacterium tuberculosis, genetic methods are being applied to identify genes that are essential to the various metabolic states that are believed to be pertinent to both the establishment and maintenance of infection.
-
The respective essential gene products are being explored using industry-validated drug-discovery methods to produce potential drug candidates. High-throughput virtual and biochemical screening efforts are described, in addition to 'lower-throughput' structure-based designs.
-
Antituberculars in clinical trials are discussed in terms of their respective modes of action and relative strengths and weaknesses as compared with current treatment modalities.
-
A select number of non-TB-approved drugs are described with regard to their potential for being applied as antituberculars. The list includes anti-infectives, such as linezolid and, perhaps surprisingly to some, anti-psychotics, such as the phenothiazine family.
-
Owing to the identification of the gene products that are essential to persistence and their new small-molecule inhibitors, which are fast acting and unencumbered by resistance, scientists may yet be able to realize the lofty goals that have been set for new antitubercular therapies.
Abstract
Tuberculosis (TB) claims a life every 10 seconds and global mortality rates are increasing despite the use of chemotherapy. But why have we not progressed towards the eradication of the disease? There is no simple answer, although apathy, politics, poverty and our inability to fight the chronic infection have all contributed. Drug resistance and HIV-1 are also greatly influencing the current TB battle plans, as our understanding of their complicity grows. In this Review, recent efforts to fight TB will be described, specifically focusing on how drug discovery could combat the resistance and persistence that make TB worthy of the moniker 'The Great White Plague'.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Boshoff, H. I. M. & Barry 3rd, C. E. Tuberculosis-metabolism and respiration in the absence of growth. Nature Rev. Microbiol. 3, 70–80 (2005).
Russell, D. G. Who puts the tubercle in tuberculosis? Nature Rev. Microbiol. 5, 39–47 (2007).
Stewart, G. R., Roberston, B. D. & Young, D. B. Tuberculosis: a problem with persistence. Nature Rev. Microbiol. 1, 97–105 (2003). Focuses on the biology of persistence in mycobacteria.
Janin, Y. L. Antituberculosis drugs: ten years of research. Bioorg. Med. Chem. 15, 2479–2513 (2007). Discusses antitubercular small molecules, from early discovery compounds to approved drugs.
Ginsberg, A. M. & Spigelman, M. Challenges in tuberculosis drug research and development. Nature Med. 13, 290–294 (2007). An enlightening discussion of the current hurdles in TB drug discovery.
Williams, K. J. & Duncan, K. Current strategies for identifying and validating targets for new treatment-shortening drugs for TB. Curr. Mol. Med. 7, 297–307 (2007). An informative exposition that focuses on drug-discovery strategies.
Hopkins, A. L. & Groom, C. R. The druggable genome. Nature Rev. Drug Discov. 1, 727–730 (2002).
Cheng, A. C. et al. Structure-based maximal affinity model predicts small-molecule druggability. Nature Biotechnol. 25, 71–75 (2007).
Boshoff, H. I. M. et al. The transcriptional responses of Mycobacterium tuberculosis to inhibitors of metabolism. J. Biol. Chem. 279, 40174–40184 (2004).
Andries, K. et al. A diarylquinoline drug active on the ATP synthase of Mycobacterium tuberculosis. Science 307, 223–227 (2005).
Manjunatha, U. H. et al. Identification of a nitroimidazo-oxazine-specific protein involved in PA-824 resistance in Mycobacterium tuberculosis. Proc. Natl Acad. Sci. USA 103, 431–436 (2006).
Camacho, L. R., Ensergueix, D., Perez, E., Gicquel, B. & Guilhot, C. Identification of a virulence gene cluster of Mycobacterium tuberculosis by signature-tagged transposon mutagenesis. Mol. Microbiol. 34, 257–267 (1999).
Cox, J. S., Chen, B., McNeil, M. & Jacobs, W. R. Complex lipid determines tissue-specific replication of Mycobacterium tuberculosis in mice. Nature 402, 79–83 (1999).
Sassetti, C. M., Boyd, D. H. & Rubin, E. J. Comprehensive identification of conditionally essential genes in mycobacteria. Proc. Natl Acad. Sci. USA 2001, 12712–12717 (2001).
Lamichane, G. et al. A postgenomic method for predicting essential genes at subsaturation levels of mutagenesis: application to Mycobacterium tuberculosis. Proc. Natl Acad. Sci. USA 12, 7213–7218 (2003).
Ehrt, S. et al. Controlling gene expression in mycobacteria with anhydrotetracycline and Tet repressor. Nucleic Acids Res. 33, e21 (2005).
Carroll, P., Muttucumaru, D. G. & Parish, T. Use of a tetracycline-inducible system for conditional expression in Mycobacterium tuberculosis and Mycobacterium smegmatis. Appl. Environ. Microbiol. 71, 3077–3084 (2005).
Blokpoel, M. C. et al. Tetracycline-inducible gene regulation in mycobacteria. Nucleic Acids Res. 33, e22 (2005).
Argyrou, A., Jin, L., Siconilfi-Baez, L., Angeletti, R. H. & Blanchard, J. S. Proteome-wide profiling of isoniazid targets in Mycobacterium tuberculosis. Biochemistry 45, 13947–13953 (2006).
Argyrou, A., Vetting, M. W., Aladegbami, B. & Blanchard, J. S. Mycobacterium tuberculosis dihydrofolate reductase is a target for isoniazid. Nature Struct. Mol. Biol. 13, 408–413 (2006).
Nopponpunth, V., Sirawaraporn, W., Greene, P. J. & Santi, D. V. Cloning and expresion of Mycobacterium tuberculosis and Mycobacterium leprae dihydropteroate synthase in Escherichia coli. J. Bacteriol. 181, 6814–6821 (1999).
Rengarajan, J. et al. The folate pathway is a target for resistance to the drug para-aminosalicyclic acid (PAS) in mycobacteria. Mol. Microbiol. 53, 275–282 (2004).
Vilcheze, C. et al. Transfer of a point mutation in Mycobacterium tuberculosis inhA resolves the target of isoniazid. Nature Med. 12, 1027–1029 (2006).
Lipinski, C. & Hopkins, A. Navigating chemical space for biology and medicine. Nature 432, 855–861 (2004).
Nwaka, S. & Hudson, A. Innovative lead discovery strategies for tropical diseases. Nature Rev. Drug Discov. 5, 941–955 (2006).
Lee, R. E. et al. Combinatorial lead optimization of [1,2]-diamines based on ethambutol as potential antituberculosis preclinical candidates. J. Comb. Chem. 5, 172–187 (2003). Demonstrates a combinatorial chemistry expansion around ethambutol that was paired with a functional-assay approach to produce novel chemical entities that modulate mycobacterial cell-wall biosynthesis, including SQ109.
Johnson, D. E. & Rodgers, A. D. Computational toxicology: heading toward more relevance in drug discovery and development. Curr. Opin. Drug Discov. Devel. 9, 29–37 (2006).
Pearl, G. M., Livingston-Carr, S. & Durham, S. K. Integration of computational analysis as a sentinel tool in toxicological assessments. Curr. Top. Med. Chem. 1, 247–255 (2001).
Goldman, R. et al. in Annual Conference on Antimicrobial Resistance (National Foundation for Infectious Diseases, Bethesda, 2006).
Alland, D., Steyn, A. J., Weisbrod, T., Aldrich, K. & Jacobs, W. R. Jr. Characterization of the Mycobacterium tuberculosis iniBAC promoter, a promoter that responds to cell wall biosynthesis inhibition. J. Bacteriol. 182, 1802–1811 (2000).
Qureshi, N., Sathyamoorthy, N. & Takayama, K. Biosynthesis of C30 to C56 fatty acids by an extract of Mycobacterium tuberculosis H37Ra. J. Bacteriol. 157, 46–52 (1984).
Rozwarski, D. A., Vilchéze, C., Sugantino, M., Bittman, R. & Sacchettini, J. C. Crystal structure of the Mycobacterium tuberculosis enoyl-ACP reductase, InhA, in complex with NAD+ and a C16 fatty acyl substrate. J. Biol. Chem. 274, 15582–15589 (1999).
Zhang, Y., Heym, B., Allen, B., Young, D. & Cole, S. The catalase-peroxidase gene and isoniazid resistance of Mycobacterium tuberculosis. Nature 358, 591–593 (1992).
Muñoz-Elías, E. J. & McKinney, J. D. Mycobacterium tuberculosis isocitrate lyases 1 and 2 are jointly required for in vivo growth and virulence. Nature Med. 11, 638–644 (2005).
Sharma, V. et al. Structure of isocitrate lyase, a persistence factor of Mycobacterium tuberculosis. Nature Struct. Biol. 7, 663–668 (2000).
White, E. L. et al. A novel inhibitor of Mycobacterium tuberculosis pantothenate synthetase. J. Biomol. Screen. 12, 100–105 (2007).
Bogatcheva, E. et al. Identification of new diamine scaffolds with activity against Mycobacterium tuberculosis. J. Med. Chem. 49, 3045–3048 (2006).
Murillo, A. C. et al. High throughput crystallography of TB drug targets. Infect. Disord. Drug Targets 7, 127–139 (2007). Discusses the use of X-ray crystallography to catalyze TB drug-discovery efforts by the elucidation of the structures of essential M. tuberculosis gene products.
Bajorath, J. Integration of virtual and high-throughput screening. Nature Rev. Drug Discov. 1, 882–894 (2002).
Shoichet, B. K. Virtual screening of chemical libraries. Nature 432, 862–865 (2004). Provides a good introduction to virtual screening using examples from drug discovery.
Shoichet, B. K., McGovern, S. L., Wei, B. & Irwin, J. J. Lead discovery using molecular docking. Curr. Opin. Chem. Biol. 6, 439–446 (2002).
Manetti, F. et al. Ligand-based virtual screening, parallel solution-phase and microwave-assisted synthesis as tools to identify and synthesize new inhibitors of Mycobacterium tuberculosis. ChemMedChem 1, 973–989 (2006).
García-García, A. et al. Search of chemical scaffolds for novel antituberculosis agents. J. Biomol. Screen. 10, 206–214 (2005).
Lipinski, C. A., Lombardo, F., Dominy, B. W. & Feeney, P. J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 23, 3–25 (1997).
Agrawal, H., Kumar, A., Bal, N. C., Siddiqi, M. I. & Arora, A. Ligand based virtual screening and biological evaluation of inhibitors of chorismate mutase (Rv1885c) from Mycobacterium tuberculosis H37Rv. Bioorg. Med. Chem. Lett. 17, 3053–3058 (2007).
Parish, T. & Stoker, N. G. The common aromatic amino acid biosynthesis pathway is essential in Mycobacterium tuberculosis. Microbiology 148, 3069–3077 (2002).
Hediger, M. E. Design, synthesis, and evaluation of aza inhibitors of chorismate mutase. Bioorg. Med. Chem. 15, 4995–5010 (2004).
Lin, T. W. et al. Structure-based inhibitor design of ACCD5, an essential acyl-CoA carboxylase carboxyltransferase domain of Mycobacterium tuberculosis. Proc. Natl Acad. Sci. USA 103, 3072–3077 (2006).
Payne, D. J., Gwynn, M. N., Holmes, D. J. & Pompliano, D. L. Drugs for bad bugs: confronting the challenges of antibacterial discovery. Nature Rev. Drug Discov. 6, 29–40 (2007).
Dessen, A., Quemard, A., Blanchard, J. S., Jacobs, W. R. Jr & Sacchettini, J. C. Crystal structure and function of the isoniazid target of M. tuberculosis. Science 267, 1638–1641 (1995).
Kuo, M. R. et al. Targeting tuberculosis and malaria through inhibition of enoyl reductase. J. Biol. Chem. 278, 20851–20859 (2003).
Rozwarski, D. A., Grant, G. A., Barton, D. H. R., Jacobs, W. R. Jr & Sacchettini, J. C. Modification of the NADH of the isoniazid target (InhA) from Mycobacterium tuberculosis. Science 279, 98–102 (1998).
Wang, F. et al. Mechanism of thionamide drug action against tuberculosis and leprosy. J. Exp. Med. 204, 73–78 (2007).
Sullivan, T. J. et al. High affinity InhA inhibitors with activity against drug-resistant strains of Mycobacterium tuberculosis. ACS Chem. Biol. 1, 43–53 (2006).
Tsukamura, M., Nakamura, E., Yoshii, S. & Amano, H. Therapeutic effect of a new antibacterial substance ofloxacin (DL8280) on pulmonary tuberculosis. Am. Rev. Respir. Dis. 131, 352–356 (1985).
Aubry, A. et al. Novel gyrase mutations in quinolone-resistant and -hypersusceptible clinical isolates of Mycobacterium tuberculosis: functional analysis of mutant enzymes. Antimicrob. Agents Chemother. 50, 104–112 (2006).
Veziris, N. et al. Treatment failure in a case of extensively drug-resistant tuberculosis associated with selection of a GyrB mutant causing fluoroquinolone resistance. Eur. J. Clin. Microbiol. Infect. Dis. 26, 423–425 (2007).
Cole, S. T. et al. Deciphering the biology of Mycobacterium tuberculosis from the complete genome sequence. Nature 393, 537–544 (1998). Determined the genome of M. tuberculosis H37Rv.
Takiff, H. E. et al. Efflux pump of the proton antiporter family confers low-level fluoroquinolone resistance in Mycobacterium smegmatis. Proc. Natl Acad. Sci. USA 9, 362–366 (1996).
Cynamon, M., Sklaney, M. R. & Shoen, C. Gatifloxacin in combination with rifampicin in a murine tuberculosis model. J. Antimicrob. Chemother. 60, 429–432 (2007).
Spigelman, M. K. New tuberculosis therapeutics: a growing pipeline. J. Infect. Dis. 196, S28–S34 (2007).
Cricchio, R., Arioli, V. & Lancini, G. C. Hydrazones of 3-formylrifamycin SV. I — hydrazones with N-amino-N′-substituted piperazines: synthesis, antibacterial activity and other biological properties. Farmaco [Sci] 30, 605–619 (1975).
Arioli, V. et al. Antibacterial activity of DL 473, a new semisynthetic rifamycin derivative. J. Antibiot. (Tokyo) 34, 1026–1032 (1981).
Bemer-Melchior, P., Bryskier, A. & Drugeon, H. B. Comparison of the in vitro activities of rifapentine and rifampicin against Mycobacterium tuberculosis. J. Antimicrob. Chemother. 46, 571–575 (2000).
Dickinson, J. M. & Mitchison, D. A. In vitro properties of rifapentine (MDL473) relevant to its use in intermittent chemotherapy of tuberculosis. Tubercle 68, 113–118 (1987).
Burman, W. J., Gallicano, K. & Peloquin, C. Comparative pharmacokinetics and pharmacodynamics of the rifamycin antibacterials. Clin. Pharmacokinet. 40, 327–341 (2001).
Yamane, T. et al. Synthesis and biological activity of 3′-hydroxy-5′-aminobenzoxazinorifamycin derivatives. Chem. Pharm. Bull. (Tokyo) 41, 148–155 (1993).
Fuji, K., Saito, H., Tomioka, H., Mae, T. & Hosoe, K. Mechanism of action of antimycobacterial activity of the new benzoxazinorifamycin KRM-1648. Antimicrob. Agents Chemother. 39, 1489–1492 (1995).
Hirata, T. et al. In vitro and in vivo activities of the benzoxazinorifamycin KRM-1648 against Mycobacterium tuberculosis. Antimicrob. Agents Chemother. 39, 2295–2303 (1995).
Klemens, S. P. & Cynamon, M. Activity of KRM-1648 in combination with isoniazid against Mycobacterium tuberculosis in a murine model. Antimicrob. Agents Chemother. 40, 298–301 (1996).
Dietze, R. et al. Safety and bactericidal activity of rifalazil in patients with pulmonary tuberculosis. Antimicrob. Agents Chemother. 45, 1972–1976 (2001).
Mae, T. et al. Effect of a new rifamycin derivative, rifalazil, on liver microsomal enzyme induction in rat and dog. Xenobiotica 28, 759–766 (1998).
Burman, W. J. & Jones, B. E. Treatment of HIV-related tuberculosis in the era of effective antiretroviral therapy. Am. J. Respir. Crit. Care Med. 164, 7–12 (2001).
Report No. TDR/PRD/TB/03.1W (World Health Organization, Geneva, 2003).
Shepherd, R. G. & Wilkinson, R. G. Antituberculous agents. II. N,N′-diisopropylethylenediamine and analogs. J. Med. Pharm. Chem. 5, 823–835 (1962).
Wilkinson, R. G., Cantrall, M. B. & Shepherd, R. G. Antituberculous agents. III. (+)-2,2-(ethylenediimino)-di-1-butanol and some analogs. J. Med. Pharm. Chem. 5, 835–845 (1962).
Jia, L. et al. Pharmacodynamics and pharmacokinetics of SQ109, a new diamine-based antitubercular drug. Br. J. Pharmacol. 144, 80–87 (2005).
Koul, A. et al. Diarylquinolines target subunit c of mycobacterial ATP synthase. Nature Chem. Biol. 3, 323–324 (2007).
Petrella, S. et al. Genetic basis for natural and acquired resistance to the diarylquinoline R207910 in mycobacteria. Antimicrob. Agents Chemother. 50, 2853–2856 (2006).
Lounis, N. et al. Combinations of R207910 with drugs used to treat multidrug-resistant tuberculosis have the potential to shorten treatment duration. Antimicrob. Agents Chemother. 50, 3543–3547 (2006).
Deidda, D. et al. Bactericidal activities of the pyrrole derivative BM212 against multidrug-resistant and intramacrophagic Mycobacterium tuberculosis strains. Antimicrob. Agents Chemother. 42, 3035–3037 (1998).
Arora, S. K., Sinha, N., Sinha, R. & Upadhyaya, R. S.U.S. Patent Application. US 2005/0256128 A1 (2005).
Casenghi, M. Development of new drugs for TB chemotherapy. Campaign for access to essential medicines [online], (2006).
Edwards, D. I. Mechanism of antimicrobial action of metronidazole. J. Antimicrob. Chemother. 5, 499–502 (1979).
Wayne, L. G. & Sohaskey, C. D. Nonreplicating persistence of Mycobacterium tuberculosis. Annu. Rev. Microbiol. 55, 139–163 (2001).
Wayne, L. G. & Sramek, H. A. Metronidazole is bactericidal to dormant cells of Mycobacterium tuberculosis. Antimicrob. Agents Chemother. 38, 2054–2058 (1994).
Brooks, J. V., Furney, S. K. & Orme, I. M. Metronidazole therapy in mice infected with tuberculosis. Antimicrob. Agents Chemother. 43, 1285–1288 (1999).
Ashtekar, D. R. et al. In vitro and in vivo activities of the nitroimidazole CGI 17341 against Mycobacterium tuberculosis. Antimicrob. Agents Chemother. 37, 183–186 (1993).
Nagrajan, K., Shankar, R. G., Rajappa, S., Shenoy, S. J. & Costa-Pereira, R. Nitroimidazoles XXI 2,3-dihydro-6-nitroimidazo [2,1-b] oxazoles with antitubercular activity. Eur. J. Med. Chem. 24, 631–633 (1989).
Stover, C. K. et al. A small-molecule nitroimidazopyran drug candidate for the treatment of tuberculosis. Nature 405, 962–966 (2000).
Tyagi, S. et al. Bactericidal activity of the nitroimidazopyran PA-824 in a murine model of tuberculosis. Antimicrob. Agents Chemother. 49, 2289–2293 (2005).
Nuermberger, E. et al. Combination chemotherapy with the nitroimidazopyran PA-824 and first-line drugs in a murine model of tuberculosis. Antimicrob. Agents Chemother. 50, 2621–2625 (2006).
Matsumoto, M. et al. OPC-67683, a nitro-dihydro-imidazooxazole derivative with promising action against tuberculosis in vitro and in mice. PLoS Med. 3, 2131–2144 (2006).
Sasaki, H. et al. Synthesis and antituberculosis activity of a novel series of optically active 6-nitro-2,3-dihydroimidazo[2,1-b]oxazoles. J. Med. Chem. 49, 7854–7860 (2006).
Raether, W. & Hänel, H. Nitroheterocyclic drugs with broad spectrum activity. Parasitol. Res. 90, S19–S39 (2003).
Brickner, S. J. et al. Synthesis and antibacterial activity of U-100592 and U-100766, two oxazolidinone antibacterial agents for the potential treatment of multidrug-resistant gram-positive bacterial infections. J. Med. Chem. 39, 673–679 (1996).
Zurenko, G. E. et al. In vitro activities of U-100592 and U-100766, novel oxazolidinone antibacterial agents. Antimicrob. Agents Chemother. 40, 839–845 (1996).
Colca, J. R. et al. Cross-linking in the living cell locates the site of action of oxazolidinone antibiotics. J. Biol. Chem. 278, 21972–21979 (2003).
Fortún, J. et al. Linezolid for the treatment of multidrug-resistant tuberculosis. J. Antimicrob. Chemother. 56, 180–185 (2005).
Ntziora, F. & Falagas, M. E. Linezolid for the treatment of patients with atypical mycobacterial infection: a systematic review. Int. J. Tuberc. Lung Dis. 11, 606–611 (2007).
Park, I. N. et al. Efficacy and tolerability of daily-half dose linezolid in patients with intractable multidrug-resistant tuberculosis. J. Antimicrob. Chemother. 58, 701–704 (2006).
von der Lippe, B., Sandven, P. & Brubakk, O. Efficacy and safety of linezolid in multidrug resistant tuberculosis (MDR-TB) — a report of ten cases. J. Infect. 52, 92–96 (2006).
Sood, R., Rao, M., Singhal, S. & Rattan, A. Activity of RBx 7644 and RBx 8700, new investigational oxazolidinones, against Mycobacterium tuberculosis infected murine macrophages. Int. J. Antimicrob. Agents 25, 464–468 (2005).
Fisher, J. F., Meroueh, S. O. & Mobashery, S. Bacterial resistance to β-lactam antibiotics: compelling opportunism, compelling opportunity. Chem. Rev. 105, 395–424 (2005).
Flores, A. R., Parsons, L. M. & Pavelka, M. S. Jr. Genetic analysis of the β-lactamases of Mycobacterium tuberculosis and Mycobacterium smegmatis and susceptibility to β-lactam antibiotics. Microbiology 151, 521–532 (2005).
Wang, F., Cassidy, C. & Sacchettini, J. C. Crystal structure and activity studies of the Mycobacterium tuberculosis β-lactamase reveal its critical role in resistance to β-lactam antibiotics. Antimicrob. Agents Chemother. 50, 2762–2771 (2006).
Flores, A. R., Parsons, L. M. & Pavelka, M. S. Jr. Characterization of novel Mycobacterium tuberculosis and Mycobacterium smegmatis mutants hypersusceptible to β-lactam antibiotics. J. Bacteriol. 187, 1892–1900 (2005).
Chambers, H. F. et al. Can penicillins and other beta-lactam antibiotics be used to treat tuberculosis? Antimicrob. Agents Chemother. 39, 2620–2624 (1995).
Chambers, H. F., Turner, J., Schecter, G. F., Kawamura, M. & Hopewell, P. C. Imipenem for treatment of tuberculosis in mice and humans. Antimicrob. Agents Chemother. 49, 2816–2821 (2005).
Rodloff, A. C., Goldstein, E. J. C. & Torres, A. Two decades of imipenem therapy. J. Antimicrob. Chemother. 58, 916–929 (2006).
Chambers, H. F., Kocagoz, S., Sipit, T., Turner, J. & Hopewell, P. C. Activity of amoxicillin/clavulanate in patients with tuberculosis. Clin. Infect. Dis. 26, 874–877 (1998).
Sauvage, E. et al. Crystal structure of the Mycobacterium fortuitum class A β-lactamase: structural basis for broad substrate specificity. Antimicrob. Agents Chemother. 50, 2516–2521 (2006).
Lopez-Munoz, F. et al. History of the discovery and clinical introduction of chlorpromazine. Ann. Clin. Psychiatry 17, 113–135 (2006).
Amaral, L., Kristiansen, J. E., Viveiros, M. & Atouguia, J. Activity of phenothiazines against antibiotic-resistant Mycobacterium tuberculosis: a review supporting further studies that may elucidate the potential use of thioridazine as anti-tuberculosis therapy. J. Antimicrob. Chemother. 47, 505–511 (2001).
Hollister, L. E., Eikenberry, D. T. & Raffel, S. Chlorpromazine in nonpsychotic patients with pulmonary tuberculosis. Am. Rev. Respir. Dis. 81, 562–566 (1960).
Amaral, L., Kristiansen, J. E., Abebe, L. S. & Millet, W. Inhibition of the respiration of multi-drug resistant clinical isolates of Mycobacterium tuberculosis by thioridazine: potential use for initial therapy of freshly diagnosed tuberculosis. J. Antimicrob. Chemother. 38, 1049–1053 (1996).
Ratnakar, P. & Murthy, P. S. Antitubercular activity of trifluoperazine, a calmodulin antagonist. FEMS Microbiol. Lett. 1, 73–76 (1992).
Reddy, M. V., Nadadhur, G. & Gangadharam, P. R. In-vitro and intracellular antimycobacterial activity of trifluoperazine. J. Antimicrob. Chemother. 37, 196–197 (1996).
Crowle, A. J., Douvas, G. S. & May, M. H. Chlorpromazine: a drug potentially useful for treating mycobacterial infections. Chemotherapy 38, 410–419 (1992).
Ordway, D. et al. Clinical concentrations of thioridazine kill intracellular multidrug-resistant Mycobacterium tuberculosis. Antimicrob. Agents Chemother. 47, 917–922 (2003).
Weinstein, E. A. et al. Inhibitors of type II NADH: menaquinone oxidoreductase represent a class of antitubercular drugs. Proc. Natl Acad. Sci. USA 102, 4548–4553 (2005).
Xie, Z., Siddiqi, N. & Rubin, E. J. Differential antibiotic susceptibilities of starved Mycobacterium tuberculosis isolates. Antimicrob. Agents Chemother. 49, 4778–4780 (2005).
Bate, A. B. et al. Synthesis and antitubercular activity of quaternized promazine and promethazine derivatives. Bioorg. Med. Chem. Lett. 17, 1346–1348 (2007).
Madrid, P. B., Polgar, W. E., Toll, L. & Tanga, M. J. Synthesis and antitubercular activity of phenothiazines with reduced binding to dopamine and serotonin receptors. Bioorg. Med. Chem. Lett. 17, 3014–3017 (2007).
Schatz, A., Bugie, E. & Waksman, S. A. Streptomycin, a substance exhibiting antibiotic activity against Gram-positive and Gram-negative bacteria. Proc. Soc. Exp. Biol. Med. 55, 66–69 (1944).
Schatz, A., Bugie, E. & Waksman, S. A. Effect of streptomycin and other antibiotic substances upon Mycobacterium tuberculosis and related organisms. Proc. Soc. Exp. Biol. Med. 57, 244–248 (1944).
Kingston, W. Streptomycin, Schatz v. Waksman, and the balance of credit for discovery. J. Hist. Med. Allied Sci. 60, 218–220 (2005).
Pettersen, E. F. et al. UCSF chimera — a visualization system for exploratory research and analysis. J. Comput. Chem. 25, 1605–1612 (2004).
Wright, D. H., Brown, G. H., Peterson, M. L. & Rotschafer, J. C. Application of fluoroquinolone pharmacodynamics. J. Antimicrob. Chemother. 46, 669–683 (2000).
McIlleron, H. et al. Determinants of rifampin, isoniazid, pyrazinamide, and ethambutol pharmacokinetics in a cohort of tuberculosis patients. Antimicrob. Agents. Chemother. 50, 1170–1177 (2006).
Berning, S. E. & Peloquin, C. A. in Antimicrobial Chemotherapy (eds Yu, V. L., Merigan, T. C., Barriere, S. & White, N. J.) 663–668 (Williams and Wilkins, Maryland, 1998).
Doluisio, J. T., Dittert, L. W. & LaPiana, J. C. Pharmacokinetics of kanamycin following intramuscular administration. J. Pharmacokinet. Biopharm. 1, 253–265 (1973).
Adamis, G. et al. Pharmacokinetic interactions of ceftazidime, imipenem and aztreonam with amikacin in healthy volunteers. Int. J. Antimicrob. Agents 23, 144–149 (2004).
Acknowledgements
The authors thank M. Spigelman, R. Goldman, J. Garcia, K. Andries, C. Dukes Hamilton, J. Guilemont, J. Johnson and A. Sternlicht for insightful conversations and, in some cases, providing unpublished results. The authors are supported by a grant from the National Institutes of Health (PO1A1068135) and the Robert A. Welch Foundation.
Author information
Authors and Affiliations
Corresponding author
Related links
Related links
DATABASES
Entrez Genome Project
FURTHER INFORMATION
James C. Sacchettini's homepage
The Merck Manuals Medical Library
Tuberculosis Antimicrobial Acquisition and Coordinating Facility
Glossary
- Pharmacokinetic profile
-
A quantitative description of the fate of a drug from the moment the treated subject is dosed with the compound to the moment when it (and/or its derivatives) is expelled from the subject.
- Pharmacophore
-
The chemical functional group (or groups) that is present on a molecule and that enables its biological activity.
- Chemotype
-
A chemical functional group or classification of a specific array of functional groups.
- Lipinski's rules
-
A set of delimited physiochemical properties described by C. A. Lipinski that best fit a studied subset of drugs. In general, compounds that adhere to these guidelines are said to be drug-like.
- Shikimate pathway
-
A series of biochemical reactions in plants and microorganisms that are involved in the biosynthesis of aromatic amino acids.
- Lead optimization
-
The process by which a promising small-molecule entity is structurally modified to obtain drug-like pharmacokinetic, pharmacodynamic and safety profiles.
- Efflux pump
-
An active transport system for the removal of toxic molecules, such as antibiotics, from cells.
- Structure–activity relationship
-
(SAR). The relationship between the chemical structure of a compound and its biological or pharmacological activity. This type of relationship can be assessed by considering a series of molecules, each with a slightly different structure, and then noting the effect on the biological activity that is associated with each structural variation.
- Fast-track status
-
The FDA status that is reserved for products that demonstrate the potential to treat a serious or life-threatening condition.
- F0 subunit of atp synthase
-
The transmembrane portion of the enzyme complex that is involved in the biosynthesis of ATP, which has a role in the passage of protons through the membrane.
- Ames mutagenicity test
-
A sensitive biological method for measuring the mutagenic potency of chemical substances.
Rights and permissions
About this article
Cite this article
Sacchettini, J., Rubin, E. & Freundlich, J. Drugs versus bugs: in pursuit of the persistent predator Mycobacterium tuberculosis. Nat Rev Microbiol 6, 41–52 (2008). https://doi.org/10.1038/nrmicro1816
Issue Date:
DOI: https://doi.org/10.1038/nrmicro1816
This article is cited by
-
BORC complex specific components and Kinesin-1 mediate autophagy evasion by the autophagy-resistant Mycobacterium tuberculosis Beijing strain
Scientific Reports (2023)
-
Evybactin is a DNA gyrase inhibitor that selectively kills Mycobacterium tuberculosis
Nature Chemical Biology (2022)
-
Short-term effect of sulfur dioxide (SO2) change on the risk of tuberculosis outpatient visits in 16 cities of Anhui Province, China: the first multi-city study to explore differences in occupational patients
Environmental Science and Pollution Research (2022)
-
Conformational analysis and quantum descriptors of two bifonazole derivatives of immense anti-tuber potential by using vibrational spectroscopy and molecular docking studies
Structural Chemistry (2021)
-
The crystal structure of mycobacterial epoxide hydrolase A
Scientific Reports (2020)