Abstract
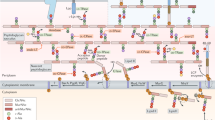
In budding yeast, the neck that connects the mother and daughter cell is the site of essential functions such as organelle trafficking, septum formation and cytokinesis. Therefore, the morphology of this region, which depends on the surrounding cell wall, must be maintained throughout the cell cycle. Growth at the neck is prevented, redundantly, by a septin ring inside the cell membrane and a chitin ring in the cell wall. Here, we describe recent work supporting the hypothesis that attachment of the chitin ring, which forms at the mother–bud neck during budding, to β-1,3-glucan in the cell wall is necessary to stop growth at the neck. Thus, in this scenario, chemistry controls morphogenesis.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Cabib, E., Roh, D.-H., Schmidt, M., Crotti, L. B. & Varma, A. The yeast cell wall and septum as paradigms of cell growth and morphogenesis. J. Biol. Chem. 276, 19679–19682 (2001).
Cid, V. J. et al. Molecular basis of cell integrity and morphogenesis in Saccharomyces cerevisiae. Microbiol. Rev. 59, 345–386 (1995).
Bi, E. & Park. H. O. Cell polarization and cytokinesis in budding yeast. Genetics 191, 347–387 (2012).
Cabib, E. The septation apparatus, a chitin-requiring machine in budding yeast. Arch. Biochem. Biophys. 426, 201–207 (2004).
Wloka, C. & Bi, E. Mechanisms of cytokinesis in budding yeast. Cytoskeleton 69, 710–726 (2012).
Hayashibe, M. & Katohda, S. Initiation of budding and chitin ring. J. Gen. Appl. Microbiol. 101, 295–301 (1973).
Cabib, E. & Bowers, B. Timing and function of chitin synthesis in yeast. J. Bacteriol. 124, 1586–1593 (1975).
Shaw, J. A. et al. The function of chitin synthase 2 and 3 in the Saccharomyces cerevisiae cell cycle. J. Cell Biol. 114, 111–123 (1991).
Molano, J., Bowers, B. & Cabib, E. Distribution of chitin in the yeast cell wall. An ultrastructural and chemical study. J. Cell Biol. 85, 199–212 (1980).
Gladfelter, A., Pringle, J. R. & Lew, D. J. The septin cortex at the yeast mother–bud neck. Curr. Opin. Microbiol. 4, 681–689 (2001).
Vrabioiu, A. M. & Mitchison, T. J. Structural insights into yeast septin organization from polarized fluorescence microscopy. Nature 443, 466–469 (2006).
Lippincott, J. & Li, R. Sequential assembly of myosin II, an IQGAP-like protein, and filamentous actin to a ring structure involved in budding yeast cytokinesis. J. Cell Biol. 140, 355–366 (1998).
Schmidt, M., Bowers, B., Varma, A., Roh, D.-H. & Cabib, E. In budding yeast, contraction of the actomyosin ring and formation of the primary septum at cytokinesis depend on each other. J. Cell Sci. 115, 293–302 (2001).
Kuranda, M. J. & Robbins, P. W. Chitinase is required for cell separation during growth of Saccharomyces cerevisiae. J. Biol. Chem. 266, 19758–19767 (1991).
Zlotnik, H., Fernández, M. P., Bowers, B. & Cabib, E. Saccharomyces cerevisiae mannoproteins form an external cell wall layer that determines wall porosity. J. Bacteriol. 159, 1018–1026 (1984).
Kollár, R., Petráková, E., Ashwell, G., Robbins, P. W. & Cabib, E. Architecture of the yeast cell wall. The linkage between chitin and β(1→3)glucan. J. Biol. Chem. 270, 1170–1178 (1995).
Kapteyn, J. C. et al. Retention of Saccharomyces cerevisiae cell wall proteins through a phosphodiester-linked β-1,3-/β-1,6-glucan heteropolymer. Glycobiology 6, 337–345 (1996).
Kollár, R. et al. Architecture of the yeast cell wall. β(1→6)glucan interconnects mannoprotein, β(1→3)glucan and chitin. J. Biol. Chem. 272, 17762–17775 (1997).
Mrsa, V. & Tanner, W. Role of NaOH-extractable cell wall proteins Ccw5p, Ccw6p, Ccw7p and Ccw8p (members of the Pir protein family) in stability of the Saccharomyces cerevisiae cell wall. Yeast 15, 813–820 (1999).
Mrsa, V., Seidl, T., Gentzch, M. & Tanner, W. Specific labeling of cell wall proteins by biotinylation. Identification of four covalently linked O-mannosylated proteins of Saccharomyces cerevisiae. Yeast 13, 1145–1154 (1997).
Ecker, M., Deutzmann, R., Lehle, L., Mrsa, V. & Tanner, W. Pir proteins of Saccharomyces cerevisiae are attached to β-1,3-glucan by a new protein-carbohydrate linkage. J. Biol. Chem. 281, 11523–11529 (2006).
Dranginis, A. M., Rauceo, J. M., Coronado, J. E. & Lipke, P. N. A biochemical guide to yeast adhesins: glycoproteins for social and antisocial occasions. Microbiol. Mol. Biol. Rev. 71, 282–294 (2007).
Cappellaro, C., Baldermann, C., Rachel, R. & Tanner, W. Mating type-specific cell-cell recognition of Saccharomyces cerevisiae: cell wall attachment and active sites of a- and alpha-agglutinin. EMBO J. 13, 4737–4744 (1994).
Mouyna, I. et al. Glycosylphosphatidylinositol-anchored glucanosyltransferases play an active role in the biosynthesis of the fungal cell wall. J. Biol. Chem. 275, 14882–14889 (2000).
Rodríguez-Peña, J. M., Cid, V. J., Arroyo, J. & Nombela, C. A novel family of cell wall-related proteins regulated differently during the yeast life cycle. Mol. Cell. Biol. 20, 3245–3255 (2000).
Cabib, E., Blanco, N., Grau, C., Rodríguez-Peña, J. M. & Arroyo, J. Crh1p and Crh2p are required for the cross-linking of chitin to β(1-6)glucan in the Saccharomyces cerevisiae cell wall. Mol. Microbiol. 63, 921–935 (2007).
Ragni, E., Sipiczki, M. & Strahl, S. Characterization of Ccw12p, a major key player in cell wall stability of Saccharomyces cerevisiae. Yeast 24, 309–319 (2007).
Orlean, P. Architecture and biosynthesis of the Saccharomyces cerevisiae cell wall. Genetics 192, 775–818 (2012).
Durán, A., Bowers, B. & Cabib, E. Chitin synthetase zymogen is attached to the yeast plasma membrane. Proc. Natl Acad. Sci. USA 72, 3952–3955 (1975).
Shematek, E. M., Braatz, J. A. & Cabib, E. Biosynthesis of the yeast cell wall. Preparation and properties of β-(1→3)glucan synthetase. J. Biol. Chem. 255, 888–894 (1980).
Cabib, E., Bowers, B. & Roberts, R. L. Vectorial synthesis of a polysaccharide by isolated plasma membranes. Proc. Natl Acad. Sci. USA 80, 3318–3321 (1983).
Lesage, G. & Bussey, H. Cell wall assembly in Saccharomyces cerevisiae. Microbiol. Mol. Biol. Rev. 70, 317–343 (2006).
Cabib, E., Bowers, B., Sburlati, A. & Silverman, S. J. Fungal wall synthesis. the construction of a biological structure. Microbiol. Sci. 5, 370–375 (1988).
Kreger, D. R. & Kopecká, M. On the nature and formation of fibrillar nets produced by protoplasts of Saccharomyces cerevisiae in liquid media: an electron microscopic, X-ray diffraction and chemical study. J. Gen. Microbiol. 92, 207–220 (1975).
Roh, D.-H., Bowers, B., Riezman, H. & Cabib, E. Rho1 mutations specific for regulation of β(1→3)glucan synthesis and the order of assembly of the yeast cell wall. Mol. Microbiol. 44, 1167–1183 (2002).
Merlini, L. & Piatti, S. The mother-bud neck as a signaling platform for the coordination between spindle position and cytokinesis in budding yeast. Biol. Chem. 392, 805–812 (2011).
Pruyne, D., Gao, L., Bi, E. & Bretscher, A. Stable and dynamic axes of polarity use distinct formin isoforms in budding yeast. Mol. Biol. Cell 15, 4971–4989 (2004).
Schmidt, M., Varma, A., Drgon, T., Bowers, B. & Cabib, E. Septins, under Cla4p regulation, and the chitin ring are required for neck integrity in budding yeast. Mol. Biol. Cell 14, 2128–2141 (2003).
Versele, M. & Thorner, J. Septin collar formation in budding yeast requires GTP binding and direct phosphorylation by the PAK, Cla4p. J. Cell Biol. 164, 701–715 (2004).
Caudron, F. & Barral, Y. Septins and the lateral compartmentation of eukaryotic membranes. Dev. Cell 16, 493–506 (2009).
Gladfelter, A. S., Bose, I., Zyla, T. R., Bardes, E. S. G. & Lew, D. J. Septin ring assembly involves cycles of GTP loading and hydrolysis by Cdc42p. J. Cell Biol. 156, 315–326 (2002).
Cabib, E. & Durán, A. Synthase III-dependent chitin is bound to different acceptors depending on location on the cell wall of budding yeast. J. Biol. Chem. 280, 9170–9179 (2005).
Cabib, E., Blanco, N. & Arroyo, J. Presence of a large β(1-3)glucan linked to chitin at the Saccharomyces cerevisiae mother-bud neck suggests involvement in localized growth control. Eukaryot. Cell 11, 388–400 (2012).
Manners, D. J., Masson, A. J. & Patterson, J. C. The structure of a β-(1→3)-D-glucan from yeast cell walls. Biochem. J. 135, 19–30 (1973).
Kopecká, M., Phaff, H. G. & Fleet, G. H. Demonstration of a fibrillar component in the cell wall of the yeast Saccharomyces cerevisiae. J. Cell Biol. 62, 66–76 (1974).
Hartland, R. P. et al. A novel β-(1-3)-glucanosyltransferase from the cell wall of Aspergillus fumigatus. J. Biol. Chem. 271, 26843–26849 (1996).
Popolo, L. & Vai, M. The Gas1 glycoprotein, a putative wall polymer cross-linker. Biochim. Biophys. Acta 1426, 385–400 (1999).
Popolo, L., Gilardelli, D., Bonfante, P. & Vai, M. Increase in chitin as an essential response to defects in assembly of cell wall polymers in the ggp1Δ mutant of Saccharomyces cerevisiae. J. Bacteriol. 179, 463–469 (1997).
Yin, Q. Y. et al. Comprehensive proteomic analysis of Saccharomyces cerevisiae cell walls. Identification of proteins covalently attached via glycosylphosphatidylinositol remnants or mild alkali-sensitive linkages. J. Biol. Chem. 280, 20894–20901 (2005).
Cabib, E. Two novel techniques for determination of polysaccharide cross-links show that Crh1p and Crh2p attach chitin to both β(1-6)- and β(1-3)glucan in the Saccharomyces cerevisiae cell wall. Eukaryot. Cell 8, 1626–1636 (2009).
Cabib, E. et al. Assembly of the yeast cell wall. Crh1p and Crh2p act as transglycosylases in vivo and in vitro. J. Biol. Chem. 283, 29859–29872 (2008).
Blanco, N., Reidy, M., Arroyo, J. & Cabib, E. Cross-links in the cell wall of budding yeast control morphogenesis at the mother–bud neck. J. Cell Sci. 125, 5781–5789 (2012).
Lew, D. J. The morphogenesis checkpoint: how yeast cells watch their figures. Curr. Opin. Cell Biol. 15, 648–653 (2003).
DeMarini, D. J. et al. A septin-based hierarchy of proteins required for localized deposition of chitin in the Saccharomyces cerevisiae cell wall. J. Cell Biol. 139, 75–93 (1997).
Kozubowski, L. et al. A Bni-Glc7 phosphatase complex that recruits chitin synthase to the site of bud emergence. Mol. Biol. Cell 14, 26–39 (2003).
Rodríguez-Peña, J. M., Rodríguez, C., Alvarez, A., Nombela, C. & Arroyo, J. Mechanism for targeting of the Saccharomyces cerevisiae GPI-anchored cell wall protein Crh2p to polarised growth sites. J. Cell Sci. 115, 2549–2558 (2002).
Dobbelaere, J. & Barral, Y. Spatial coordination of cytokinetic events by compartmentalization of the cell cortex. Science 305, 393–396 (2004).
Oh, Y. & Bi, E. Septin structure and function in yeast and beyond. Trends Cell Biol. 21, 141–148 (2011).
Bertin, A. et al. Three-dimensional ultrastructure of the septin filament network in Saccharomyces cerevisiae. Mol. Biol. Cell 23, 423–432 (2012).
Richman, T. J., Sawyer, M. M. & Johnson, D. I. The Cdc42p GTPase is involved in a G2/M morphogenetic checkpoint regulating the apical-isotropic switch and nuclear division in yeast. J. Biol. Chem. 274, 16861–16870 (1999).
Wessels, J. G. H. Cell wall synthesis in apical hyphal growth. Int. Rev. Cytol. 104, 37–79 (1986).
Fayant, P. et al. Finite element model of polar growth of pollen tubes. Plant Cell 22, 2579–2593 (2010).
Wolf, S., Hématy, K. & Höfte, H. Growth control and cell wall signaling in plants. Annu. Rev. Plant Biol. 63, 381–407 (2012).
Hayashi, T. & Kaida, R. Functions of xyloglucan in plant cells. Mol. Plant 4, 17–24 (2011).
Hrmova, M., Farkas, V., Lahnstein, J. & Fincher, G. B. A barley xyloglucan xyloglucosyl transferase covalently links xyloglucan, cellulosic substrates, and (1,3;1,4)-β-D-glucans. J. Biol. Chem. 282, 12951–12962 (2007).
Cavalier, D. M. et al. Disrupting two Arabidopsis thaliana xylosyltransferase genes results in plants deficient in xyloglucan, a major primary cell wall component. Plant Cell 20, 1519–1537 (2008).
Acknowledgements
The authors thank V. Cid, O. Cohen-Fix, P. Pérez, M. Reidy and C. Vázquez de Aldana for critical reading of the manuscript, and R. García for help with the figures. E.C. was supported by a US National Institutes of Health grant (as part of the Intramural Research Program of the National Institutes of Diabetes and Digestive and Kidney Diseases). J.A. acknowledges all the members of his laboratory for their dedicated work, and C. Nombela and the Special Chair in Genomics and Proteomics for their support. Research in the J.A. laboratory is supported by the Spanish Government Ministry of Economy and Competitiveness (grant BIO2010-22146), the Universidad Complutense de Madrid (grant GR58/08), the Comunidad de Madrid (grant S2010/BDM-2414) and the European Science Foundation (grant 06-RNP-132).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Cabib, E., Arroyo, J. How carbohydrates sculpt cells: chemical control of morphogenesis in the yeast cell wall. Nat Rev Microbiol 11, 648–655 (2013). https://doi.org/10.1038/nrmicro3090
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrmicro3090
This article is cited by
-
Saccharomyces cerevisiae supplemented diets mitigate the effects of waterborne cadmium toxicity on gilthead seabream (Sparus aurata L.): growth performance, haemato-biochemical, stress biomarkers, and histopathological investigations
Veterinary Research Communications (2024)
-
The β-1,3-glucan synthase gene GFGLS2 plays major roles in mycelial growth and polysaccharide synthesis in Grifola frondosa
Applied Microbiology and Biotechnology (2022)
-
The Saccharomyces cerevisiae Ncw2 protein works on the chitin/β-glucan organisation of the cell wall
Antonie van Leeuwenhoek (2021)
-
Chitin synthase localization in the diatom Thalassiosira pseudonana
BMC Materials (2020)
-
Antifungal peptides produced by actinomycetes and their biological activities against plant diseases
The Journal of Antibiotics (2020)