Key Points
-
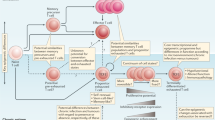
T cells exposed to persistent antigen and/or inflammatory signals in chronic infection or cancer can become 'exhausted', a state characterized by a hierarchical loss of effector functions and memory T cell properties, and by the expression of multiple inhibitory receptors.
-
T cell exhaustion prevents optimal control of infections and tumours, but modulating inhibitory pathways that are overexpressed in exhaustion can reverse this dysfunctional state and reinvigorate immune responses.
-
Exhausted T cells are a distinct lineage of differentiated T cells; these cells are phenotypically and mechanistically different from other dysfunctional states of T cells such as anergy and senescence.
-
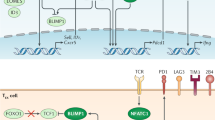
Altered usage of transcription factors is a key feature of T cell exhaustion. Whereas transcription factors such as T-bet, EOMES (eomesodermin) and BLIMP1 (B lymphocyte-induced maturation protein 1) can have roles in other T cell populations, their expression pattern, target genes and functions in exhausted T cells are distinct.
-
Antigen-specific CD4+ T cells also progress to exhaustion during chronic infection. Although CD4+ and CD8+ T cell exhaustion share a core transcriptional signature, exhaustion of CD4+ T cells is distinct from that of CD8+ T cells, as each subset has different expression patterns of molecules such as inhibitory receptors and transcription factors.
Abstract
In chronic infections and cancer, T cells are exposed to persistent antigen and/or inflammatory signals. This scenario is often associated with the deterioration of T cell function: a state called 'exhaustion'. Exhausted T cells lose robust effector functions, express multiple inhibitory receptors and are defined by an altered transcriptional programme. T cell exhaustion is often associated with inefficient control of persisting infections and tumours, but revitalization of exhausted T cells can reinvigorate immunity. Here, we review recent advances that provide a clearer molecular understanding of T cell exhaustion and reveal new therapeutic targets for persisting infections and cancer.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Kaech, S. M. & Cui, W. Transcriptional control of effector and memory CD8+ T cell differentiation. Nat. Rev. Immunol. 12, 749–761 (2012).
Masopust, D. & Schenkel, J. M. The integration of T cell migration, differentiation and function. Nat. Rev. Immunol. 13, 309–320 (2013).
Wherry, E. J. T cell exhaustion. Nat. Immunol. 131, 492–499 (2011).
Doering, T. A. et al. Network analysis reveals centrally connected genes and pathways involved in CD8+ T cell exhaustion versus memory. Immunity 37, 1130–1144 (2012).This study shows that memory and exhausted CD8+ T cells have partially non-overlapping modules and centrally connected genes that are thought to be the hubs or foci of biological processes. This reference also indicates that transcription factors have distinct connections in exhausted CD8+ T cells compared with memory CD8+ T cells.
Schietinger, A. & Greenberg, P. D. Tolerance and exhaustion: defining mechanisms of T cell dysfunction. Trends Immunol. 35, 51–60 (2014).
Zajac, A. J. et al. Viral immune evasion due to persistence of activated T cells without effector function. J. Exp. Med. 188, 2205–2213 (1998).
Gallimore, A. et al. Induction and exhaustion of lymphocytic choriomeningitis virus-specific cytotoxic T lymphocytes visualized using soluble tetrameric major histocompatibility complex class I-peptide complexes. J. Exp. Med. 187, 1383–1393 (1998). References 6 and 7 describe T cell exhaustion as the dysfunction and subsequent physical deletion of antigen-specific T cells during chronic viral infection.
Barber, D. L. et al. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature 439, 682–687 (2006). This study shows that exhausted CD8+ T cells can be reinvigorated by blocking PD1 signalling during chronic viral infection in mice.
Nguyen, L. T. & Ohashi, P. S. Clinical blockade of PD1 and LAG3 — potential mechanisms of action. Nat. Rev. Immunol. 15, 45–56 (2014).
Pardoll, D. M. The blockade of immune checkpoints in cancer immunotherapy. Nat. Rev. Cancer 12, 252–264 (2012).
Pauken, K. E. & Wherry, E. J. Overcoming T cell exhaustion in infection and cancer. Trends Immunol. 36, 265–276 (2015).
Paley, M. A. et al. Progenitor and terminal subsets of CD8+ T cells cooperate to contain chronic viral infection. Science 338, 1220–1225 (2012). This study shows the lineage relationships, hierarchy and cooperative maintenance of subpopulations of exhausted CD8+ T cells during chronic infection.
Matloubian, M., Concepcion, R. J. & Ahmed, R. CD4+ T cells are required to sustain CD8+ cytotoxic T-cell responses during chronic viral infection. J. Virol. 68, 8056–8063 (1994). This study shows that CD4+ T cell help is also required to sustain CD8+ T cell responses during chronic viral infection.
Quigley, M. et al. Transcriptional analysis of HIV-specific CD8+ T cells shows that PD-1 inhibits T cell function by upregulating BATF. Nat. Med. 16, 1147–1151 (2010).
Wherry, E. J., Blattman, J. N., Murali-Krishna, K., van der Most, R. & Ahmed, R. Viral persistence alters CD8 T-cell immunodominance and tissue distribution and results in distinct stages of functional impairment. J. Virol. 77, 4911–4927 (2003).
Bucks, C. M., Norton, J. A., Boesteanu, A. C., Mueller, Y. M. & Katsikis, P. D. Chronic antigen stimulation alone is sufficient to drive CD8+ T cell exhaustion. J. Immunol. 182, 6697–6708 (2009).
Streeck, H. et al. Antigen load and viral sequence diversification determine the functional profile of HIV-1-specific CD8+ T cells. PLoS Med. 5, e100 (2008).
Blackburn, S. D. et al. Coregulation of CD8+ T cell exhaustion by multiple inhibitory receptors during chronic viral infection. Nat. Immunol. 10, 29–37 (2009). This study shows that inhibitory receptors are co-expressed by exhausted T cells, that their expression is correlated with the severity of T cell exhaustion, and that co-blockade of multiple inhibitory receptors synergistically reinvigorates exhausted T cells.
Brooks, D. G., McGavern, D. B. & Oldstone, M. B. Reprogramming of antiviral T cells prevents inactivation and restores T cell activity during persistent viral infection. J. Clin. Invest. 116, 1675–1685 (2006).
Angelosanto, J. M., Blackburn, S. D., Crawford, A. & Wherry, E. J. Progressive loss of memory T cell potential and commitment to exhaustion during chronic viral infection. J. Virol. 86, 8161–8170 (2012).
Kasprowicz, V. et al. Hepatitis C virus (HCV) sequence variation induces an HCV-specific T-cell phenotype analogous to spontaneous resolution. J. Virol. 84, 1656–1663 (2010).
Agnellini, P. et al. Impaired NFAT nuclear translocation results in split exhaustion of virus-specific CD8+ T cell functions during chronic viral infection. Proc. Natl Acad. Sci. USA 104, 4565–4570 (2007).
Chiu, Y. L. et al. Sprouty-2 regulates HIV-specific T cell polyfunctionality. J. Clin. Invest. 124, 198–208 (2014).
Martinez, G. J. et al. The transcription factor NFAT promotes exhaustion of activated CD8+ T cells. Immunity 42, 265–278 (2015). This study shows that AP-1-independent NFAT activity promotes T cell anergy and exhaustion.
Oestreich, K. J., Yoon, H., Ahmed, R. & Boss, J. M. NFATc1 regulates PD-1 expression upon T cell activation. J. Immunol. 181, 4832–4839 (2008).
Honda, T. et al. Tuning of antigen sensitivity by T cell receptor-dependent negative feedback controls T cell effector function in inflamed tissues. Immunity 40, 235–247 (2014).
Okazaki, T., Chikuma, S., Iwai, Y., Fagarasan, S. & Honjo, T. A rheostat for immune responses: the unique properties of PD-1 and their advantages for clinical application. Nat. Immunol. 14, 1212–1218 (2013).
Sharpe, A. H., Wherry, E. J., Ahmed, R. & Freeman, G. J. The function of programmed cell death 1 and its ligands in regulating autoimmunity and infection. Nat. Immunol. 8, 239–245 (2007).
Odorizzi, P. M. & Wherry, E. J. Inhibitory receptors on lymphocytes: insights from infections. J. Immunol. 188, 2957–2965 (2012).
Araki, K., Youngblood, B. & Ahmed, R. Programmed cell death 1-directed immunotherapy for enhancing T-cell function. Cold Spring Harb. Symp. Quant. Biol. 78, 239–247 (2013).
Pentcheva-Hoang, T., Egen, J. G., Wojnoonski, K. & Allison, J. P. B7-1 and B7-2 selectively recruit CTLA-4 and CD28 to the immunological synapse. Immunity 21, 401–413 (2004).
Parry, R. V. et al. CTLA-4 and PD-1 receptors inhibit T-cell activation by distinct mechanisms. Mol. Cell. Biol. 25, 9543–9553 (2005).
Yokosuka, T. et al. Programmed cell death 1 forms negative costimulatory microclusters that directly inhibit T cell receptor signaling by recruiting phosphatase SHP2. J. Exp. Med. 209, 1201–1217 (2012).
Clayton, K. L. et al. T cell Ig and mucin domain-containing protein 3 is recruited to the immune synapse, disrupts stable synapse formation, and associates with receptor phosphatases. J. Immunol. 192, 782–791 (2014).
Riley, J. L. PD-1 signaling in primary T cells. Immunol. Rev. 229, 114–125 (2009).
Chemnitz, J. M., Parry, R. V., Nichols, K. E., June, C. H. & Riley, J. L. SHP-1 and SHP-2 associate with immunoreceptor tyrosine-based switch motif of programmed death 1 upon primary human T cell stimulation, but only receptor ligation prevents T cell activation. J. Immunol. 173, 945–954 (2004).
Patsoukis, N. et al. Selective effects of PD-1 on Akt and Ras pathways regulate molecular components of the cell cycle and inhibit T cell proliferation. Sci. Signal. 5, ra46 (2012).
Patsoukis, N., Sari, D. & Boussiotis, V. A. PD-1 inhibits T cell proliferation by upregulating p27 and p15 and suppressing Cdc25A. Cell Cycle 11, 4305–4309 (2012).
Zinselmeyer, B. H. et al. PD-1 promotes immune exhaustion by inducing antiviral T cell motility paralysis. J. Exp. Med. 210, 757–774 (2013).
Duraiswamy, J. et al. Phenotype, function, and gene expression profiles of programmed death-1hi CD8 T cells in healthy human adults. J. Immunol. 186, 4200–4212 (2011).
Dolfi, D. V. et al. Increased T-bet is associated with senescence of influenza virus-specific CD8 T cells in aged humans. J. Leukoc. Biol. 93, 825–836 (2013).
Blackburn, S. D., Shin, H., Freeman, G. J. & Wherry, E. J. Selective expansion of a subset of exhausted CD8 T cells by anti-PD-L1 blockade. Proc. Natl Acad. Sci. USA 105, 15016–15021 (2008). This study identifies subsets of exhausted CD8+ T cells and shows that exhausted CD8+ T cells with intermediate expression of PD1 can be reinvigorated by PD1 blockade, whereas a PD1hi subset cannot.
Day, C. L. et al. PD-1 expression on HIV-specific T cells is associated with T-cell exhaustion and disease progression. Nature 443, 350–354 (2006).
Blattman, J. N., Wherry, E. J., Ha, S. J., van der Most, R. G. & Ahmed, R. Impact of epitope escape on PD-1 expression and CD8 T-cell exhaustion during chronic infection. J. Virol. 83, 4386–4394 (2009).
Utzschneider, D. T. et al. T cells maintain an exhausted phenotype after antigen withdrawal and population reexpansion. Nat. Immunol. 14, 603–610 (2013).
Kaufmann, D. E. et al. Upregulation of CTLA-4 by HIV-specific CD4+ T cells correlates with disease progression and defines a reversible immune dysfunction. Nat. Immunol. 8, 1246–1254 (2007).
Butler, N. S. et al. Therapeutic blockade of PD-L1 and LAG-3 rapidly clears established blood-stage Plasmodium infection. Nat. Immunol. 13, 188–195 (2011).
Grosso, J. F. et al. Functionally distinct LAG-3 and PD-1 subsets on activated and chronically stimulated CD8 T cells. J. Immunol. 182, 6659–6669 (2009).
Matsuzaki, J. et al. Tumor-infiltrating NY-ESO-1-specific CD8+ T cells are negatively regulated by LAG-3 and PD-1 in human ovarian cancer. Proc. Natl Acad. Sci. USA 107, 7875–7880 (2010).
Jin, H. T. et al. Cooperation of Tim-3 and PD-1 in CD8 T-cell exhaustion during chronic viral infection. Proc. Natl Acad. Sci. USA 107, 14733–14738 (2010).
Kassu, A. et al. Regulation of virus-specific CD4+ T cell function by multiple costimulatory receptors during chronic HIV infection. J. Immunol. 185, 3007–3018 (2010).
Sakuishi, K. et al. Targeting Tim-3 and PD-1 pathways to reverse T cell exhaustion and restore anti-tumor immunity. J. Exp. Med. 207, 2187–2194 (2010).
Fourcade, J. et al. Upregulation of Tim-3 and PD-1 expression is associated with tumor antigen-specific CD8+ T cell dysfunction in melanoma patients. J. Exp. Med. 207, 2175–2186 (2010).
Wolchok, J. D. et al. Nivolumab plus ipilimumab in advanced melanoma. N. Engl. J. Med. 369, 122–133 (2013). This study shows that co-blockade of PD1 and CTLA4 in humans leads to impressive tumour regression compared with monotherapy in patients with melanoma.
Wang, C. et al. Loss of the signaling adaptor TRAF1 causes CD8+ T cell dysregulation during human and murine chronic infection. J. Exp. Med. 209, 77–91 (2012).
Vezys, V. et al. 4-1BB signaling synergizes with programmed death ligand 1 blockade to augment CD8 T cell responses during chronic viral infection. J. Immunol. 187, 1634–1642 (2011).
Brooks, D. G. et al. Interleukin-10 determines viral clearance or persistence in vivo. Nat. Med. 12, 1301–1309 (2006).
Ejrnaes, M. et al. Resolution of a chronic viral infection after interleukin-10 receptor blockade. J. Exp. Med. 203, 2461–2472 (2006). References 57 and 58 show that the immunoregulatory cytokine IL-10 has an important role in promoting or sustaining T cell exhaustion.
Ng, C. T. & Oldstone, M. B. Infected CD8α− dendritic cells are the predominant source of IL-10 during establishment of persistent viral infection. Proc. Natl Acad. Sci. USA 109, 14116–14121 (2012).
Said, E. A. et al. Programmed death-1-induced interleukin-10 production by monocytes impairs CD4+ T cell activation during HIV infection. Nat. Med. 16, 452–459 (2010).
Richter, K. et al. Macrophage and T cell produced IL-10 promotes viral chronicity. PLoS Pathog. 9, e1003735 (2013).
Parish, I. A. et al. Chronic viral infection promotes sustained Th1-derived immunoregulatory IL-10 via BLIMP-1. J. Clin. Invest. 124, 3455–3468 (2014).
Brooks, D. G. et al. IL-10 and PD-L1 operate through distinct pathways to suppress T-cell activity during persistent viral infection. Proc. Natl Acad. Sci. USA 105, 20428–20433 (2008).
Tinoco, R., Alcalde, V., Yang, Y., Sauer, K. & Zuniga, E. I. Cell-intrinsic transforming growth factor-β signaling mediates virus-specific CD8+ T cell deletion and viral persistence in vivo. Immunity 31, 145–157 (2009).
Garidou, L., Heydari, S., Gossa, S. & McGavern, D. B. Therapeutic blockade of transforming growth factor beta fails to promote clearance of a persistent viral infection. J. Virol. 86, 7060–7071 (2012).
Boettler, T., Cheng, Y., Ehrhardt, K. & von Herrath, M. TGF-β blockade does not improve control of an established persistent viral infection. Viral Immunol. 25, 232–238 (2012).
Sandler, N. G. et al. Type I interferon responses in rhesus macaques prevent SIV infection and slow disease progression. Nature 511, 601–605 (2014).
Curtsinger, J. M. & Mescher, M. F. Inflammatory cytokines as a third signal for T cell activation. Curr. Opin. Immunol. 22, 333–340 (2010).
Teijaro, J. R. et al. Persistent LCMV infection is controlled by blockade of type I interferon signaling. Science 340, 207–211 (2013).
Wilson, E. B. et al. Blockade of chronic type I interferon signaling to control persistent LCMV infection. Science 340, 202–207 (2013). References 69 and 70 show that while acutely antiviral, sustained type I IFN activity has detrimental effects on antiviral T cell immunity and paradoxically fosters viral persistance and T cell exhaustion.
Ivashkiv, L. B. & Donlin, L. T. Regulation of type I interferon responses. Nat. Rev. Immunol. 14, 36–49 (2014).
Stelekati, E. et al. Bystander chronic infection negatively impacts development of CD8+ T cell memory. Immunity 40, 801–813 (2014).
Harker, J. A., Lewis, G. M., Mack, L. & Zuniga, E. I. Late interleukin-6 escalates T follicular helper cell responses and controls a chronic viral infection. Science 334, 825–829 (2011).
Harker, J. A., Dolgoter, A. & Zuniga, E. I. Cell-intrinsic IL-27 and gp130 cytokine receptor signaling regulates virus-specific CD4+ T cell responses and viral control during chronic infection. Immunity 39, 548–559 (2013).
Belkaid, Y. & Tarbell, K. Regulatory T cells in the control of host-microorganism interactions. Annu. Rev. Immunol. 27, 551–589 (2009).
Veiga-Parga, T., Sehrawat, S. & Rouse, B. T. Role of regulatory T cells during virus infection. Immunol. Rev. 255, 182–196 (2013).
Penaloza-MacMaster, P. et al. Interplay between regulatory T cells and PD-1 in modulating T cell exhaustion and viral control during chronic LCMV infection. J. Exp. Med. 211, 1905–1918 (2014).
Ng, C. T., Snell, L. M., Brooks, D. G. & Oldstone, M. B. Networking at the level of host immunity: immune cell interactions during persistent viral infections. Cell Host Microbe 13, 652–664 (2013).
Goh, C., Narayanan, S. & Hahn, Y. S. Myeloid-derived suppressor cells: the dark knight or the joker in viral infections? Immunol. Rev. 255, 210–221 (2013).
Waggoner, S. N., Cornberg, M., Selin, L. K. & Welsh, R. M. Natural killer cells act as rheostats modulating antiviral T cells. Nature 481, 394–398 (2012).
Holderried, T. A., Lang, P. A., Kim, H. J. & Cantor, H. Genetic disruption of CD8+ TReg activity enhances the immune response to viral infection. Proc. Natl Acad. Sci. USA 110, 21089–21094 (2013).
Joosten, S. A. et al. Identification of a human CD8+ regulatory T cell subset that mediates suppression through the chemokine CC chemokine ligand 4. Proc. Natl Acad. Sci. USA 104, 8029–8034 (2007).
Sevilla, N., McGavern, D. B., Teng, C., Kunz, S. & Oldstone, M. B. A. Viral targeting of hematopoietic progenitors and inhibition of DC maturation as a dual strategy for immune subversion. J. Clin. Invest. 113, 737–745 (2004). This study shows that DCs can be infected by LCMV and that altered DC development and/or function is associated with reduced antigen presentation and with T cell dysfunction.
Mueller, S. N. et al. Viral targeting of fibroblastic reticular cells contributes to immunosuppression and persistence during chronic infection. Proc. Natl Acad. Sci. USA 104, 15430–15435 (2007).
Schacker, T. The role of secondary lymphatic tissue in immune deficiency of HIV infection. AIDS 22 (Suppl. 3), 13–18 (2008).
Zeng, M. et al. Cumulative mechanisms of lymphoid tissue fibrosis and T cell depletion in HIV-1 and SIV infections. J. Clin. Invest. 121, 998–1008 (2011).
Elsaesser, H., Sauer, K. & Brooks, D. G. IL-21 is required to control chronic viral infection. Science 324, 1569–1572 (2009).
Frohlich, A. et al. IL-21R on T cells is critical for sustained functionality and control of chronic viral infection. Science 324, 1576–1580 (2009).
Yi, J. S., Du, M. & Zajac, A. J. A vital role for interleukin-21 in the control of a chronic viral infection. Science 324, 1572–1576 (2009). References 87–89 show that IL-21 produced by antigen-specific CD4+ T cells during chronic LCMV infection is needed to sustain antiviral CD8+ T cell responses.
Williams, L. D. et al. Interleukin-21-producing HIV-1-specific CD8 T cells are preferentially seen in elite controllers. J. Virol. 85, 2316–2324 (2011).
Chevalier, M. F. et al. HIV-1-specific interleukin-21+ CD4+ T cell responses contribute to durable viral control through the modulation of HIV-specific CD8+ T cell function. J. Virol. 85, 733–741 (2011).
Ackerman, M. E. et al. Natural variation in Fc glycosylation of HIV-specific antibodies impacts antiviral activity. J. Clin. Invest. 123, 2183–2192 (2013).
Straub, T. et al. Nucleoprotein-specific nonneutralizing antibodies speed up LCMV elimination independently of complement and FcγR. Eur. J. Immunol. 43, 2338–2348 (2013).
Richter, K. & Oxenius, A. Non-neutralizing antibodies protect from chronic LCMV infection independently of activating FcγR or complement. Eur. J. Immunol. 43, 2349–2360 (2013).
Fahey, L. M. et al. Viral persistence redirects CD4 T cell differentiation toward T follicular helper cells. J. Exp. Med. 208, 987–999 (2011).
Crawford, A. et al. Molecular and transcriptional basis of CD4+ T cell dysfunction during chronic infection. Immunity 40, 289–302 (2014).
Wherry, E. J. et al. Molecular signature of CD8+ T cell exhaustion during chronic viral infection. Immunity 27, 670–684 (2007).
Intlekofer, A. M. et al. Effector and memory CD8+ T cell fate coupled by T-bet and eomesodermin. Nat. Immunol. 6, 1236–1244 (2005).
Brenchley, J. M. et al. Expression of CD57 defines replicative senescence and antigen-induced apoptotic death of CD8+ T cells. Blood 101, 2711–2720 (2003).
Akbar, A. N. & Henson, S. M. Are senescence and exhaustion intertwined or unrelated processes that compromise immunity? Nat. Rev. Immunol. 11, 289–295 (2011).
Wirth, T. C. et al. Repetitive antigen stimulation induces stepwise transcriptome diversification but preserves a core signature of memory CD8+ T cell differentiation. Immunity 33, 128–140 (2010).
Schluns, K. S. & Lefrancois, L. Cytokine control of memory T-cell development and survival. Nat. Rev. Immunol. 3, 269–279 (2003).
Wherry, E. J., Barber, D. L., Kaech, S. M., Blattman, J. N. & Ahmed, R. Antigen-independent memory CD8 T cells do not develop during chronic viral infection. Proc. Natl Acad. Sci. USA 101, 16004–16009 (2004).
Shin, H., Blackburn, S. D., Blattman, J. N. & Wherry, E. J. Viral antigen and extensive division maintain virus-specific CD8 T cells during chronic infection. J. Exp. Med. 204, 941–949 (2007).
Garcia, F. et al. The virological and immunological consequences of structured treatment interruptions in chronic HIV-1 infection. AIDS 15, F29–F40 (2001).
Ortiz, G. M. et al. Structured antiretroviral treatment interruptions in chronically HIV-1-infected subjects. Proc. Natl Acad. Sci. USA 98, 13288–13293 (2001).
Altfeld, M. et al. Expansion of pre-existing, lymph node-localized CD8+ T cells during supervised treatment interruptions in chronic HIV-1 infection. J. Clin. Invest. 109, 837–843 (2002).
Alter, G. et al. Longitudinal assessment of changes in HIV-specific effector activity in HIV-infected patients starting highly active antiretroviral therapy in primary infection. J. Immunol. 171, 477–488 (2003).
Jamieson, B. D. et al. Epitope escape mutation and decay of human immunodeficiency virus type 1-specific CTL responses. J. Immunol. 171, 5372–5379 (2003).
Obar, J. J., Crist, S. G., Leung, E. K. & Usherwood, E. J. IL-15-independent proliferative renewal of memory CD8+ T cells in latent gammaherpesvirus infection. J. Immunol. 173, 2705–2714 (2004).
Snyder, C. M. et al. Memory inflation during chronic viral infection is maintained by continuous production of short-lived, functional T cells. Immunity 29, 650–659 (2008).
Blattman, J. N. et al. Therapeutic use of IL-2 to enhance antiviral T-cell responses in vivo. Nat. Med. 9, 540–547 (2003).
West, E. E. et al. PD-L1 blockade synergizes with IL-2 therapy in reinvigorating exhausted T cells. J. Clin. Invest. 123, 2604–2615 (2013).
Pellegrini, M. et al. IL-7 engages multiple mechanisms to overcome chronic viral infection and limit organ pathology. Cell 144, 601–613 (2011).
Nanjappa, S. G., Kim, E. H. & Suresh, M. Immunotherapeutic effects of IL-7 during a chronic viral infection in mice. Blood 117, 5123–5132 (2011).
Schmitz, I. et al. IL-21 restricts virus-driven TReg cell expansion in chronic LCMV infection. PLoS Pathog. 9, e1003362 (2013).
Kao, C. et al. Transcription factor T-bet represses expression of the inhibitory receptor PD-1 and sustains virus-specific CD8+ T cell responses during chronic infection. Nat. Immunol. 12, 663–671 (2011). This study shows that the transcription factor T-bet is differentially used in memory T cells compared with exhausted T cells and that T-bet directly represses expression of the gene encoding PD1.
Buggert, M. et al. T-bet and Eomes are differentially linked to the exhausted phenotype of CD8+ T cells in HIV infection. PLoS Pathog. 10, e1004251 (2014).
Vezys, V. et al. Continuous recruitment of naive T cells contributes to heterogeneity of antiviral CD8 T cells during persistent infection. J. Exp. Med. 203, 2263–2269 (2006).
Wilson, J. J. et al. CD8 T cells recruited early in mouse polyomavirus infection undergo exhaustion. J. Immunol. 188, 4340–4348 (2012).
Miller, N. E., Bonczyk, J. R., Nakayama, Y. & Suresh, M. Role of thymic output in regulating CD8 T-cell homeostasis during acute and chronic viral infection. J. Virol. 79, 9419–9429 (2005).
Youngblood, B. et al. Chronic virus infection enforces demethylation of the locus that encodes PD-1 in antigen-specific CD8+ T cells. Immunity 35, 400–412 (2011). This study provides initial insights into the epigenetic changes that underlie T cell exhaustion.
Shin, H. et al. A role for the transcriptional repressor Blimp-1 in CD8+ T cell exhaustion during chronic viral infection. Immunity 31, 309–320 (2009).
Doedens, A. L. et al. Hypoxia-inducible factors enhance the effector responses of CD8+ T cells to persistent antigen. Nat. Immunol. 14, 1173–1182 (2013).
Staron, M. M. et al. The transcription factor FoxO1 sustains expression of the inhibitory receptor PD-1 and survival of antiviral CD8+ T cells during chronic infection. Immunity 41, 802–814 (2014).
Stephen, T. L. et al. Transforming growth factor β-mediated suppression of antitumour T cells requires FoxP1 transcription factor expression. Immunity 41, 427–439 (2014).
Intlekofer, A. M. et al. Requirement for T-bet in the aberrant differentiation of unhelped memory CD8+ T cells. J. Exp. Med. 204, 2015–2021 (2007).
Paley, M. A. et al. Technical advance: fluorescent reporter reveals insights into eomesodermin biology in cytotoxic lymphocytes. J. Leukoc. Biol. 93, 307–315 (2013).
Banerjee, A. et al. Cutting edge: the transcription factor eomesodermin enables CD8+ T cells to compete for the memory cell niche. J. Immunol. 185, 4988–4992 (2010).
Zhou, X. et al. Differentiation and persistence of memory CD8+ T cells depend on T cell factor 1. Immunity 33, 229–240 (2010).
Mullen, A. C. et al. Master transcription factors determine cell-type-specific responses to TGF-β signaling. Cell 147, 565–576 (2011).
Trompouki, E. et al. Lineage regulators direct BMP and Wnt pathways to cell-specific programs during differentiation and regeneration. Cell 147, 577–589 (2011).
Youngblood, B. et al. Cutting edge: prolonged exposure to HIV reinforces a poised epigenetic program for PD-1 expression in virus-specific CD8 T cells. J. Immunol. 191, 540–544 (2013).
Zhang, F. et al. Epigenetic manipulation restores functions of defective CD8+ T cells from chronic viral infection. Mol. Ther. 22, 1698–1706 (2014).
Nishimura, T. et al. Generation of rejuvenated antigen-specific T cells by reprogramming to pluripotency and redifferentiation. Cell Stem Cell 12, 114–126 (2013).
Pearce, E. L., Poffenberger, M. C., Chang, C. H. & Jones, R. G. Fueling immunity: insights into metabolism and lymphocyte function. Science 342, 1242454 (2013).
Schmitz, J. E. et al. Control of viremia in simian immunodeficiency virus infection by CD8+ lymphocytes. Science 283, 857–860 (1999).
Jin, X. et al. Dramatic rise in plasma viremia after CD8+ T cell depletion in simian immunodeficiency virus-infected macaques. J. Exp. Med. 189, 991–998 (1999).
Cornberg, M. et al. Clonal exhaustion as a mechanism to protect against severe immunopathology and death from an overwhelming CD8 T cell response. Front. Immunol. 4, 475 (2013).
Khanna, K. M., Lepisto, A. J., Decman, V. & Hendricks, R. L. Immune control of herpes simplex virus during latency. Curr. Opin. Immunol. 16, 463–469 (2004).
Frank, G. M. et al. Early CD4+ T cell help prevents partial CD8+ T cell exhaustion and promotes maintenance of herpes simplex virus 1 latency. J. Immunol. 184, 277–286 (2010).
Oxenius, A., Zinkernagel, R. M. & Hengartner, H. Comparison of activation versus induction of unresponsiveness of virus-specific CD4+ and CD8+ T cells upon acute versus persistent viral infection. Immunity 9, 449–457 (1998).
Brooks, D. G., Teyton, L., Oldstone, M. B. & McGavern, D. B. Intrinsic functional dysregulation of CD4 T cells occurs rapidly following persistent viral infection. J. Virol. 79, 10514–10527 (2005).
Morou, A., Palmer, B. E. & Kaufmann, D. E. Distinctive features of CD4+ T cell dysfunction in chronic viral infections. Curr. Opin. HIV AIDS 9, 446–451 (2014).
Schulze Zur Wiesch, J. et al. Broadly directed virus-specific CD4+ T cell responses are primed during acute hepatitis C infection, but rapidly disappear from human blood with viral persistence. J. Exp. Med. 209, 61–75 (2012).
Osokine, I. et al. Type I interferon suppresses de novo virus-specific CD4 Th1 immunity during an established persistent viral infection. Proc. Natl Acad. Sci. USA 111, 7409–7414 (2014).
Charles, E. D. & Dustin, L. B. Hepatitis C virus-induced cryoglobulinemia. Kidney Int. 76, 818–824 (2009).
Haas, A., Zimmermann, K. & Oxenius, A. Antigen-dependent and -independent mechanisms of T and B cell hyperactivation during chronic HIV-1 infection. J. Virol. 85, 12102–12113 (2011).
Hunziker, L. et al. Hypergammaglobulinemia and autoantibody induction mechanisms in viral infections. Nat. Immunol. 4, 343–349 (2003).
Acknowledgements
The authors thank the members of Wherry laboratory for discussions. In particular, they would like to thank J. Kurachi for technical assistance and figure preparation, and E. Stelekati and K. Pauken for helpful comments. The authors' work is supported by The Ministry of Education, Culture, Sports, Science and Technology of Japan (Grants-in-Aid for Young Scientists (B) 22790453 to M.K.), the Uehara Memorial Foundation of Japan (M.K.) and the US National Institutes of Health (grants AI105343, AI112521, AI082630, AI095608 and HHSN266200500030C to E.J.W.).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
E.J.W. has a patent licensing agreement with Roche/Genentech on the PD1 pathway and is a member of the scientific advisory board of Surface Oncology. M.K. declares no competing interests.
Related links
FURTHER INFORMATION
Glossary
- Effector memory T cells
-
A subset of memory T cells that resides in peripheral tissues and retains high levels of effector function such as the production of interferon-γ and granzymes.
- Central memory T cells
-
A subset of memory T cells that expresses CD62L and CC-chemokine receptor 7 and resides mainly in the lymph nodes and spleen. These cells have high proliferative potential upon antigen re-encounter.
- Lymphocytic choriomeningitis virus
-
(LCMV). A virus that induces a strong T cell response and therefore provides good mouse model of infection. Although they only differ by two nucleotides, the Armstrong strain of LCMV causes acute infection and the clone 13 strain causes chronic infection. As major antigenic epitopes are conserved between these two strains, the fate of antigen-specific T cells can be easily compared (that is, memory after infection with the Armstrong strain versus exhaustion after infection with the clone 13 strain).
- Antigen-presenting cells
-
(APCs). T cells can be activated when antigen is displayed by major histocompatibility complexes (MHC restriction). This antigen presentation is largely executed by professional APCs, particularly dendritic cells. APCs also influence T cell differentiation by producing cytokines such as type I interferons and interleukin-12.
- Senescent T cells
-
Cells that enter a terminal differentiation state owing to excessive cell replication. This state is associated with irreversible cell cycle arrest and telomere shortening.
- Anergic T cells
-
An unresponsive state that is induced by suboptimal stimulation (that is, signal 1 without signal 2) at the time of priming by antigen.
- Epigenetic landscape
-
Historically, this is proposed to refer to embryonic development and how genes might interact with their surroundings to lead to the expression of a certain phenotype. The current view of the epigenetic landscape is that overall epigenetic changes such as (but not limited to) DNA methylation and histone modifications, are accumulated genome wide and have strong impact on cellular differentiation.
Rights and permissions
About this article
Cite this article
Wherry, E., Kurachi, M. Molecular and cellular insights into T cell exhaustion. Nat Rev Immunol 15, 486–499 (2015). https://doi.org/10.1038/nri3862
Published:
Issue Date:
DOI: https://doi.org/10.1038/nri3862
This article is cited by
-
Upregulation of PD-1/PD-L1 and downregulation of immune signaling pathways lead to more severe visceral leishmaniasis in undernutrition mice
Parasites & Vectors (2024)
-
BATF and BATF3 deficiency alters CD8+ effector/exhausted T cells balance in skin transplantation
Molecular Medicine (2024)
-
The immune checkpoint TIGIT/CD155 promotes the exhaustion of CD8 + T cells in TNBC through glucose metabolic reprogramming mediated by PI3K/AKT/mTOR signaling
Cell Communication and Signaling (2024)
-
Targeted deletion of CD244 on monocytes promotes differentiation into anti-tumorigenic macrophages and potentiates PD-L1 blockade in melanoma
Molecular Cancer (2024)
-
Per-cell histone acetylation is associated with terminal differentiation in human T cells
Clinical Epigenetics (2024)