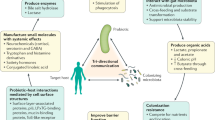
Abstract
The essential role of the gut microbiota for health has generated tremendous interest in modulating its composition and metabolic function. One of these strategies is prebiotics, which typically refer to selectively fermented nondigestible food ingredients or substances that specifically support the growth and/or activity of health-promoting bacteria that colonize the gastrointestinal tract. In this Perspective, we argue that advances in our understanding of diet–microbiome–host interactions challenge important aspects of the current concept of prebiotics, and especially the requirement for effects to be 'selective' or 'specific'. We propose to revise this concept in an effort to shift the focus towards ecological and functional features of the microbiota more likely to be relevant for host physiology. This revision would provide a more rational basis for the identification of prebiotic compounds, and a framework by which the therapeutic potential of modulating the gut microbiota could be more fully materialized.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
Change history
23 April 2015
In the version of this article originally published online a statement about antibiotics in relation to the 2008 FAO definition of prebiotics was incorrect in Table 1 and has now been deleted. The error has been corrected for the print, HTML and PDF versions of the article.
References
Brestoff, J. R. & Artis, D. Commensal bacteria at the interface of host metabolism and the immune system. Nat. Immunol. 14, 676–684 (2013).
Belkaid, Y. & Hand, T. W. Role of the microbiota in immunity and inflammation. Cell 157, 121–141 (2014).
Britton, R. A. & Young, V. B. Role of the intestinal microbiota in resistance to colonization by Clostridium difficile. 146, 1547–1553 (2014).
Haag, L. M. et al. Intestinal microbiota shifts towards elevated commensal Escherichia coli loads abrogate colonization resistance against Campylobacter jejuni in mice. PLoS ONE 7, e35988 (2012).
Kostic, A. D., Xavier, R. J. & Gevers, D. The microbiome in inflammatory bowel disease: current status and the future ahead. Gastroenterology 146, 1489–1499 (2014).
Manichanh, C., Borruel, N., Casellas, F. & Guarner, F. The gut microbiota in IBD. Nat. Rev. Gastroenterol. Hepatol. 9, 599–608 (2012).
Louis, P., Hold, G. L. & Flint, H. J. The gut microbiota, bacterial metabolites and colorectal cancer. Nat. Rev. Microbiol. 12, 661–672 (2014).
Schwabe, R. F. & Jobin, C. The microbiome and cancer. Nat. Rev. Cancer 13, 800–812 (2013).
Cox, L. M. & Blaser, M. J. Pathways in microbe-induced obesity. Cell Metab. 17, 883–894 (2013).
Khan, M. T., Nieuwdorp, M. & Backhed, F. Microbial modulation of insulin sensitivity. Cell Metab. 20, 753–760 (2014).
Zhao, L. The gut microbiota and obesity: from correlation to causality. Nat. Rev. Microbiol. 11, 639–647 (2013).
Delzenne, N. M., Neyrinck, A. M. & Cani, P. D. Gut microbiota and metabolic disorders: how prebiotic can work? Br. J. Nutr. 109 (Suppl. 2), S81–S85 (2013).
Tilg, H. & Moschen, A. R. Microbiota and diabetes: an evolving relationship. 63, 1513–1521 (2014).
Backhed, F., Manchester, J. K., Semenkovich, C. F. & Gordon, J. I. Mechanisms underlying the resistance to diet-induced obesity in germ-free mice. Proc. Natl Acad. Sci. USA 104, 979–984 (2007).
Hashimoto, T. et al. ACE2 links amino acid malnutrition to microbial ecology and intestinal inflammation. Nature 487, 477 (2012).
Smith, M. I. et al. Gut microbiomes of Malawian twin pairs discordant for kwashiorkor. Science 339, 548–554 (2013).
Wang, Z. et al. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature 472, 57–63 (2011).
Wu, H. J. et al. Gut-residing segmented filamentous bacteria drive autoimmune arthritis via T helper 17 cells. Immunity 32, 815–827 (2010).
Brusca, S. B., Abramson, S. B. & Scher, J. U. Microbiome and mucosal inflammation as extra-articular triggers for rheumatoid arthritis and autoimmunity. Curr. Opin. Rheumatol. 26, 101–107 (2014).
Ramezani, A. & Raj, D. S. The gut microbiome, kidney disease, and targeted interventions. J. Am. Soc. Nephrol. 25, 657–670 (2014).
Berer, K. et al. Commensal microbiota and myelin autoantigen cooperate to trigger autoimmune demyelination. Nature 479, 538–541 (2011).
Noval Rivas, M. et al. A microbiota signature associated with experimental food allergy promotes allergic sensitization and anaphylaxis. J. Allergy Clin. Immunol. 131, 201–212 (2013).
Ridaura, V. K. et al. Gut microbiota from twins discordant for obesity modulate metabolism in mice. Science 341, 1241214 (2013).
Arthur, J. C. et al. Microbial genomic analysis reveals the essential role of inflammation in bacteria-induced colorectal cancer. Nat. Commun. 5, 4724 (2014).
Walker, A. W. & Lawley, T. D. Therapeutic modulation of intestinal dysbiosis. Pharmacol. Res. 69, 75–86 (2013).
Olle, B. Medicines from microbiota. Nat. Biotechnol. 31, 309–315 (2013).
Flint, H. J., Duncan, S. H., Scott, K. P. & Louis, P. Links between diet, gut microbiota composition and gut metabolism. Proc. Nutr. Soc. 74, 13–22 (2015).
Metchnikoff, E. The Prolongation of Life: Optimistic Studies (ed. Chalmers Mitchell, P.). (G. P. Putnam's Sons, 1908).
Roberfroid, M. et al. Prebiotic effects: metabolic and health benefits. Br. J. Nutr. 104 (Suppl. 2), S1–S63 (2010).
Leach, J. D. Evolutionary perspective on dietary intake of fibre and colorectal cancer. Eur. J. Clin. Nutr. 61, 140–142 (2007).
Arpaia, N. et al. Metabolites produced by commensal bacteria promote peripheral regulatory T-cell generation. Nature 504, 451–455 (2013).
Trompette, A. et al. Gut microbiota metabolism of dietary fiber influences allergic airway disease and hematopoiesis. Nat. Med. 20, 159–166 (2014).
Bindels, L. B. et al. Gut microbiota-derived propionate reduces cancer cell proliferation in the liver. Br. J. Cancer 107, 1337–1344 (2012).
De Vadder, F. et al. Microbiota-generated metabolites promote metabolic benefits via gut–brain neural circuits. Cell 156, 84–96 (2014).
Gao, Z. et al. Butyrate improves insulin sensitivity and increases energy expenditure in mice. Diabetes 58, 1509–1517 (2009).
Furusawa, Y. et al. Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature 504, 446–450 (2013).
Gibson, G. R. & Roberfroid, M. B. Dietary modulation of the human colonic microbiota: introducing the concept of prebiotics. J. Nutr. 125, 1401–1412 (1995).
Rastall, R. A. & Gibson, G. R. Recent developments in prebiotics to selectively impact beneficial microbes and promote intestinal health. Curr. Opin. Biotechnol. 32C, 42–46 (2014).
Nyangale, E. P., Mottram, D. S. & Gibson, G. R. Gut microbial activity, implications for health and disease: the potential role of metabolite analysis. J. Proteome. Res. 11, 5573–5585 (2012).
Schrezenmeir, J. & de Vrese, M. Probiotics, prebiotics, and synbiotics—approaching a definition. Am. J. Clin. Nutr. 73 (2 Suppl), 361S–364S (2001).
Reid, G. et al. New scientific paradigms for probiotics and prebiotics. J. Clin. Gastroenterol. 37, 105–118 (2003).
Kaczmarczyk, M. M., Miller, M. J. & Freund, G. G. The health benefits of dietary fiber: beyond the usual suspects of type 2 diabetes mellitus, cardiovascular disease and colon cancer. Metabolism 61, 1058–1066 (2012).
Slavin, J. Fiber and prebiotics: mechanisms and health benefits. Nutrients 5, 1417–1435 (2013).
Jakobsdottir, G., Nyman, M. & Fak, F. Designing future prebiotic fiber to target metabolic syndrome. Nutrition 30, 497–502 (2014).
Holscher, H. D. et al. Fiber supplementation influences phylogenetic structure and functional capacity of the human intestinal microbiome: follow-up of a randomized controlled trial. Am. J. Clin. Nutr. 101, 55–64 (2015).
Human Microbiome Project Consortium et al. Structure, function and diversity of the healthy human microbiome, Nature 486, 207–214 (2012).
Le Chatelier, E. et al. Richness of human gut microbiome correlates with metabolic markers. Nature 500, 541–546 (2013).
Guarner, F. Decade in review—gut microbiota: The gut microbiota era marches on. Nat. Rev. Gastroenterol. Hepatol. 11, 647–649 (2014).
Qin, J. et al. A human gut microbial gene catalogue established by metagenomic sequencing. Nature 464, 59–65 (2010).
Gill, S. R. et al. Metagenomic analysis of the human distal gut microbiome. Science 312, 1355–1359 (2006).
FAO. FAO Technical Meeting on Prebiotics, Rome. Advance Analytical Technologies [online], (2008).
Gibson, G. R. et al. Dietary prebiotics: current status and new definition. Food Science and Technology Bulletin: Functional Foods 7, 1–19 (2010).
Atarashi, K. et al. Treg induction by a rationally selected mixture of Clostridia strains from the human microbiota. Nature 500, 232–236 (2013).
Sokol, H. et al. Faecalibacterium prausnitzii is an anti-inflammatory commensal bacterium identified by gut microbiota analysis of Crohn disease patients. Proc. Natl Acad. Sci. USA 105, 16731–16736 (2008).
Everard, A. et al. Responses of gut microbiota and glucose and lipid metabolism to prebiotics in genetic obese and diet-induced leptin-resistant mice. Diabetes 60, 2775–2786 (2011).
Everard, A. et al. Cross-talk between Akkermansia muciniphila and intestinal epithelium controls diet-induced obesity. Proc. Natl Acad. Sci. USA 110, 9066–9071 (2013).
Moran, J. P., Walter, J., Tannock, G. W., Tonkonogy, S. L. & Sartor, R. B. Bifidobacterium animalis causes extensive duodenitis and mild colonic inflammation in monoassociated interleukin-10-deficient mice. Inflamm. Bowel Dis. 15, 1022–1031 (2009).
Veiga, P. et al. Bifidobacterium animalis subsp. lactis fermented milk product reduces inflammation by altering a niche for colitogenic microbes. Proc. Natl Acad. Sci. USA 107, 18132–18127 (2010).
Costello, E. K., Stagaman, K., Dethlefsen, L., Bohannan, B. J. & Relman, D. A. The application of ecological theory toward an understanding of the human microbiome. Science 336, 1255–1262 (2012).
Lawley, T. D. & Walker, A. W. Intestinal colonization resistance. Immunology 138, 1–11 (2013).
van Nood, E. et al. Duodenal infusion of donor feces for recurrent Clostridium difficile. N. Engl. J. Med. 368, 407–415 (2013).
Vrieze, A. et al. Transfer of intestinal microbiota from lean donors increases insulin sensitivity in individuals with metabolic syndrome. Gastroenterology 143, 913–916 (2012).
Bindels, L. B., Dewulf, E. M. & Delzenne, N. M. GPR43/FFA2: physiopathological relevance and therapeutic prospects. Trends Pharmacol. Sci. 34, 226–232 (2013).
Fukuda, S. et al. Bifidobacteria can protect from enteropathogenic infection through production of acetate. Nature 469, 543–547 (2011).
Reichardt, N. et al. Phylogenetic distribution of three pathways for propionate production within the human gut microbiota. ISME J. 8, 1323–1335 (2014).
Louis, P., Young, P., Holtrop, G. & Flint, H. J. Diversity of human colonic butyrate-producing bacteria revealed by analysis of the butyryl-CoA:acetate CoA-transferase gene. Environ. Microbiol. 12, 304–314 (2010).
Hamaker, B. R. & Tuncil, Y. E. A perspective on the complexity of dietary fiber structures and their potential effect on the gut microbiota. J. Mol. Biol. 426, 3838–3850 (2014).
Birt, D. F. et al. Resistant starch: promise for improving human health. Adv. Nutr. 4, 587–601 (2013).
Robertson, M. D. Dietary-resistant starch and glucose metabolism. Curr. Opin. Clin. Nutr. Metab Care 15, 362–367 (2012).
Gibson, G. R., Beatty, E. R., Wang, X. & Cummings, J. H. Selective stimulation of bifidobacteria in the human colon by oligofructose and inulin. Gastroenterology 108, 975–982 (1995).
Roberfroid, M. Prebiotics: the concept revisited. J. Nutr. 137 (3 Suppl. 2), 830S–837S (2007).
Everard, A. et al. Microbiome of prebiotic-treated mice reveals novel targets involved in host response during obesity. ISME J. 8, 2116–2130 (2014).
Dewulf, E. M. et al. Insight into the prebiotic concept: lessons from an exploratory, double blind intervention study with inulin-type fructans in obese women. Gut 62, 1112–1121 (2012).
Davis, L. M., Martinez, I., Walter, J., Goin, C. & Hutkins, R. W. Barcoded pyrosequencing reveals that consumption of galactooligosaccharides results in a highly specific bifidogenic response in humans. PLoS ONE 6, e25200 (2011).
Ladirat, S. E. et al. Exploring the effects of galacto-oligosaccharides on the gut microbiota of healthy adults receiving amoxicillin treatment. Br. J. Nutr. 112, 536–546 (2014).
Maathuis, A. J., van den Heuvel, E. G., Schoterman, M. H. & Venema, K. Galacto-oligosaccharides have prebiotic activity in a dynamic in vitro colon model using a 13C-labeling technique. J. Nutr. 142, 1205–1212 (2012).
Serino, M. et al. Metabolic adaptation to a high-fat diet is associated with a change in the gut microbiota. Gut 61, 543–553 (2012).
Furuse, S. U. et al. Galacto-oligosaccharides attenuate renal injury with microbiota modification. Physiol. Rep. 2, e12029 (2014).
Nakamizo, S. et al. Commensal bacteria and cutaneous immunity. Semin. Immunopathol. 37, 73–80 (2014).
Maruyama, N. et al. Intraindividual variation in core microbiota in peri-implantitis and periodontitis. Sci. Rep. 4, 6602 (2014).
Reid, G. Probiotic and prebiotic applications for vaginal health. J. AOAC Int. 95, 31–34 (2012).
Wade, W. G. The oral microbiome in health and disease. Pharmacol. Res. 69, 137–143 (2013).
Cox, L. M. et al. The nonfermentable dietary fiber hydroxypropyl methylcellulose modulates intestinal microbiota. FASEB J. 27, 692–702 (2013).
Cani, P. D. et al. Changes in gut microbiota control inflammation in obese mice through a mechanism involving GLP-2-driven improvement of gut permeability. Gut 58, 1091–1103 (2009).
Quintero, M. et al. Adherence inhibition of Cronobacter sakazakii to intestinal epithelial cells by prebiotic oligosaccharides. Curr. Microbiol. 62, 1448–1454 (2011).
Zenhom, M. et al. Prebiotic oligosaccharides reduce proinflammatory cytokines in intestinal Caco-2 cells via activation of PPARγ and peptidoglycan recognition protein 3. J. Nutr. 141, 971–977 (2011).
Hassaninasab, A., Hashimoto, Y., Tomita-Yokotani, K. & Kobayashi, M. Discovery of the curcumin metabolic pathway involving a unique enzyme in an intestinal microorganism. Proc. Natl Acad. Sci. USA 108, 6615–6620 (2011).
van Duynhoven, J. et al. Metabolic fate of polyphenols in the human superorganism. Proc. Natl Acad. Sci. USA 108 (Suppl. 1), 4531–4538 (2011).
Bolca, S., Van de Wiele, T. & Possemiers, S. Gut metabotypes govern health effects of dietary polyphenols. Curr. Opin. Biotechnol. 24, 220–225 (2013).
Anhe, F. F. et al. A polyphenol-rich cranberry extract protects from diet-induced obesity, insulin resistance and intestinal inflammation in association with increased Akkermansia spp. population in the gut microbiota of mice. Gut http://dx.doi.org/10.1136/gutjnl-2014-307142.
Neyrinck, A. M. et al. Polyphenol-rich extract of pomegranate peel alleviates tissue inflammation and hypercholesterolaemia in high-fat diet-induced obese mice: potential implication of the gut microbiota. Br. J. Nutr. 109, 802–809 (2013).
Bereswill, S. et al. Anti-inflammatory effects of resveratrol, curcumin and simvastatin in acute small intestinal inflammation. PLoS ONE 5, e15099 (2010).
Viaud, S. et al. The intestinal microbiota modulates the anticancer immune effects of cyclophosphamide. Science 342, 971–976 (2013).
Shin, N. R. et al. An increase in the Akkermansia spp. population induced by metformin treatment improves glucose homeostasis in diet-induced obese mice. Gut 63, 727–735 (2014).
Zhang, X. et al. Structural changes of gut microbiota during berberine-mediated prevention of obesity and insulin resistance in high-fat diet-fed rats. PLoS ONE 7, e42529 (2012).
Sanders, M. E. et al. Health claims substantiation for probiotic and prebiotic products. Gut Microbes 2, 127–133 (2011).
Tachon, S., Zhou, J., Keenan, M., Martin, R. & Marco, M. L. The intestinal microbiota in aged mice is modulated by dietary resistant starch and correlated with improvements in host responses. FEMS Microbiol. Ecol. 83, 299–309 (2013).
Martinez, I. et al. Gut microbiome composition is linked to whole grain-induced immunological improvements. ISME J. 7, 269–280 (2013).
Pacheco, A. R., Barile, D., Underwood, M. A. & Mills, D. A. The impact of the milk glycobiome on the neonate gut microbiota. Annu. Rev. Anim. Biosci. 3, 419–445 (2014).
Neyrinck, A. M. et al. Prebiotic effects of wheat arabinoxylan related to the increase in bifidobacteria, Roseburia and Bacteroides/Prevotella in diet-induced obese mice. PLoS ONE 6, e20944 (2011).
Walter, J., Martinez, I. & Rose, D. J. Holobiont nutrition: considering the role of the gastrointestinal microbiota in the health benefits of whole grains. Gut Microbes 4, 340–346 (2013).
Fremont-Rahl, J. J. et al. An analysis of the role of the indigenous microbiota in cholesterol gallstone pathogenesis. PLoS ONE 8, e70657 (2013).
Respondek, F. et al. Short-chain fructo-oligosaccharides modulate intestinal microbiota and metabolic parameters of humanized gnotobiotic diet induced obesity mice. PLoS ONE 8, e71026 (2013).
Hill, C. et al. Expert consensus document: the International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 11, 506–514 (2014).
Gibson, G. R., Probert, H. M., Loo, J. V., Rastall, R. A. & Roberfroid, M. B. Dietary modulation of the human colonic microbiota: updating the concept of prebiotics. Nutr. Res. Rev. 17, 259–275 (2004).
Acknowledgements
L.B.B and P.D.C. are, respectively, Postdoctoral Researcher and Research Associate from the F.R.S.-FNRS (Fond National de la Recherche Scientifique, Belgium). P.D.C. is the recipient of an European Research Council Starting Grant 2013 (Starting grant 336452-ENIGMO), FNRS subsidies (credit de recherche convention J.0084.15 and convention 3.4579.11, projet de recherches T0.138.14; Fonds de la recherche scientifique, Belgium), grants from FRFS-WELBIO (WELBIO-CR-2012S-02R) and ARC (Concerted Research Activities-French Community of Belgium convention: 12/17-047). N.M.D. is the recipient of grants from the Région Wallonne (Programme d'excellence 2013, FOOD4GUT), the European Union's Seventh Framework Program (KBBE.2013.2.2-02 MyNewGut project) and the F.R.S-F.N.R.S (CDR J.0122.15). J.W. acknowledges start-up funds from the University of Alberta and thanks S. Loehr (University of Alberta, Canada) for critical reading of the manuscript.
Author information
Authors and Affiliations
Contributions
L.B.B and J.W. researched data for and wrote the article. All authors made substantial contributions to discussion of content and reviewed/edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
PowerPoint slides
Rights and permissions
About this article
Cite this article
Bindels, L., Delzenne, N., Cani, P. et al. Towards a more comprehensive concept for prebiotics. Nat Rev Gastroenterol Hepatol 12, 303–310 (2015). https://doi.org/10.1038/nrgastro.2015.47
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrgastro.2015.47
This article is cited by
-
Synbiotics as potent functional food: recent updates on therapeutic potential and mechanistic insight
Journal of Food Science and Technology (2024)
-
Impact of Dietary Probiotics on the Immune and Reproductive Physiology of Pubertal Male Japanese Quail (Coturnix coturnix japonica) Administered at the Onset of Pre-Puberty
Probiotics and Antimicrobial Proteins (2024)
-
Bacillus coagulans MZY531 alleviates intestinal mucosal injury in immunosuppressive mice via modulating intestinal barrier, inflammatory response, and gut microbiota
Scientific Reports (2023)
-
Identifying glycan consumers in human gut microbiota samples using metabolic labeling coupled with fluorescence-activated cell sorting
Nature Communications (2023)
-
Utilization of diverse oligosaccharides for growth by Bifidobacterium and Lactobacillus species and their in vitro co-cultivation characteristics
International Microbiology (2023)